Integrin Regulators in Neutrophils
- PMID: 35805108
- PMCID: PMC9266208
- DOI: 10.3390/cells11132025
Integrin Regulators in Neutrophils
Abstract
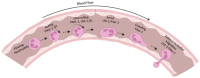
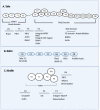
Neutrophils are the most abundant leukocytes in humans and are critical for innate immunity and inflammation. Integrins are critical for neutrophil functions, especially for their recruitment to sites of inflammation or infections. Integrin conformational changes during activation have been heavily investigated but are still not fully understood. Many regulators, such as talin, Rap1-interacting adaptor molecule (RIAM), Rap1, and kindlin, are critical for integrin activation and might be potential targets for integrin-regulating drugs in treating inflammatory diseases. In this review, we outline integrin activation regulators in neutrophils with a focus on the above critical regulators, as well as newly discovered modulators that are involved in integrin activation.
Keywords: RIAM; Rap1; integrins; kindlin; neutrophils; talin.
Conflict of interest statement
No conflict of interest, financial or otherwise, are declared by the authors.
Figures

Similar articles
-
The Rap1-RIAM-talin axis of integrin activation and blood cell function.Blood. 2016 Jul 28;128(4):479-87. doi: 10.1182/blood-2015-12-638700. Epub 2016 May 20. Blood. 2016. PMID: 27207789 Free PMC article. Review.
-
Structural and mechanistic insights into the recruitment of talin by RIAM in integrin signaling.Structure. 2014 Dec 2;22(12):1810-1820. doi: 10.1016/j.str.2014.09.020. Epub 2014 Nov 20. Structure. 2014. PMID: 25465129 Free PMC article.
-
RIAM activates integrins by linking talin to ras GTPase membrane-targeting sequences.J Biol Chem. 2009 Feb 20;284(8):5119-27. doi: 10.1074/jbc.M807117200. Epub 2008 Dec 19. J Biol Chem. 2009. PMID: 19098287 Free PMC article.
-
Loss of the Rap1 effector RIAM results in leukocyte adhesion deficiency due to impaired β2 integrin function in mice.Blood. 2015 Dec 17;126(25):2704-12. doi: 10.1182/blood-2015-05-647453. Epub 2015 Sep 3. Blood. 2015. PMID: 26337492 Free PMC article.
-
Structural, biochemical, and functional properties of the Rap1-Interacting Adaptor Molecule (RIAM).Biomed J. 2022 Apr;45(2):289-298. doi: 10.1016/j.bj.2021.09.005. Epub 2021 Oct 1. Biomed J. 2022. PMID: 34601137 Free PMC article. Review.
Cited by
-
Biodegradable exosome-engineered hydrogels for the prevention of peritoneal adhesions via anti-oxidation and anti-inflammation.Mater Today Bio. 2024 Oct 24;29:101312. doi: 10.1016/j.mtbio.2024.101312. eCollection 2024 Dec. Mater Today Bio. 2024. PMID: 39525394 Free PMC article.
-
The Rac-GEF Tiam1 controls integrin-dependent neutrophil responses.Front Immunol. 2023 Nov 21;14:1223653. doi: 10.3389/fimmu.2023.1223653. eCollection 2023. Front Immunol. 2023. PMID: 38077328 Free PMC article.
-
Genetic ablation of myeloid integrin α9 attenuates early atherosclerosis.J Leukoc Biol. 2024 Nov 4;116(5):1208-1214. doi: 10.1093/jleuko/qiae161. J Leukoc Biol. 2024. PMID: 39036986 Free PMC article.
-
Relationship between selenium status, selenoproteins and COVID-19 and other inflammatory diseases: A critical review.J Trace Elem Med Biol. 2023 Jan;75:127099. doi: 10.1016/j.jtemb.2022.127099. Epub 2022 Nov 3. J Trace Elem Med Biol. 2023. PMID: 36372013 Free PMC article. Review.
-
Monitoring Circulating Myeloid Cells in Peritonitis with an In Vivo Imaging Flow Cytometer.Biomolecules. 2024 Jul 23;14(8):886. doi: 10.3390/biom14080886. Biomolecules. 2024. PMID: 39199274 Free PMC article.
References
-
- Drummond R.A., Collar A.L., Swamydas M., Rodriguez C.A., Lim J.K., Mendez L.M., Fink D.L., Hsu A.P., Zhai B., Karauzum H., et al. CARD9-Dependent Neutrophil Recruitment Protects against Fungal Invasion of the Central Nervous System. PLoS Pathog. 2015;11:e1005293. doi: 10.1371/journal.ppat.1005293. - DOI - PMC - PubMed
-
- Saitoh T., Komano J., Saitoh Y., Misawa T., Takahama M., Kozaki T., Uehata T., Iwasaki H., Omori H., Yamaoka S., et al. Neutrophil Extracellular Traps Mediate a Host Defense Response to Human Immunodeficiency Virus-1. Cell Host Microbe. 2012;12:109–116. doi: 10.1016/j.chom.2012.05.015. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

