Eimeria falciformis secretes extracellular vesicles to modulate proinflammatory response during interaction with mouse intestinal epithelial cells
- PMID: 35804396
- PMCID: PMC9270845
- DOI: 10.1186/s13071-022-05364-x
Eimeria falciformis secretes extracellular vesicles to modulate proinflammatory response during interaction with mouse intestinal epithelial cells
Abstract
Background: Protozoan parasite secretions can be triggered by various modified media and diverse physicochemical stressors. Equally, host-parasite interactions are known to co-opt the exchange and secretion of soluble biochemical components. Analysis of Eimeria falciformis sporozoite secretions in response to interaction with mouse intestinal epithelial cells (MIECs) may reveal parasite secretory motifs, protein composition and inflammatory activities of E. falciformis extracellular vesicles (EVs).
Methods: Eimeria falciformis sporozoites were allowed to interact with inactivated MIECs. Parasite secretions were separated into EV and vesicle-free (VF) fractions by discontinuous centrifugation and ultracentrifugation. Secreted EVs were purified in an iodixanol density gradient medium and the protein composition of both EV and VF fractions were analyzed by liquid chromatoraphy-tandem mass spectroscopy. The inflammatory activities of E. falciformis sporozoite EV on MIECs were then investigated.
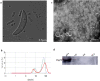
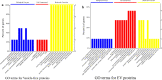
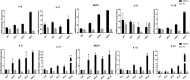
Results: During the interaction of E. falciformis sporozoites with inactivated MIECs, the parasite secreted VF and vesicle-bound molecules. Eimeria falciformis vesicles are typical pathogenic protozoan EVs with a mean diameter of 264 ± 2 nm, and enclosed heat shock protein (Hsp) 70 as classical EV marker. Refractile body-associated aspartyl proteinase (or eimepsin), GAP45 and aminopeptidase were the main components of E. falciformis sporozoite EVs, while VF proteins include Hsp90, actin, Vps54 and kinases, among others. Proteomic data revealed that E. falciformis EV and VF proteins are aggregates of bioactive, antigenic and immunogenic molecules which act in concert for E. falciformis sporozoite motility, pathogenesis and survival. Moreover, in MIECs, E. falciformis EVs induced upregulation of gene expression and secretion of IL-1β, IL-6, IL-17, IL-18, MCP1 as well as pyroptosis-dependent caspase 11 and NLRP6 inflammasomes with the concomitant secretion of lactate dehydrogenase.
Conclusions: Eimeria falciformis sporozoite interaction with MIECs triggered the secretion of immunogenic and antigenic proteins. In addition, E. falciformis sporozoite EVs constitute parasite-associated molecular pattern that induced inflammatory response and cell death. This study offers additional insight in the secretion and protein composition of E. falciformis secretomes as well as the proinflammatory functions of E. falciformis sporozoite EVs.
Keywords: Eimeria falciformis; Extracellular vesicles; Host-parasite interactions; Inflammasomes; Pyroptosis; Secretome.
© 2022. The Author(s).
Conflict of interest statement
All authors declare that there is no conflict of interest.
Figures

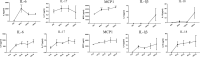
Similar articles
-
Eimeria falciformis extracellular vesicles differentially express host cell lncRNAs.J Eukaryot Microbiol. 2024 Mar-Apr;71(2):e13009. doi: 10.1111/jeu.13009. Epub 2023 Dec 10. J Eukaryot Microbiol. 2024. PMID: 38073253
-
Quantitative proteomic analysis of local and systemic extracellular vesicles during Eimeria falciformis infectious cycle in the host.Parasit Vectors. 2023 Sep 27;16(1):339. doi: 10.1186/s13071-023-05906-x. Parasit Vectors. 2023. PMID: 37759313 Free PMC article.
-
The genome of Eimeria falciformis--reduction and specialization in a single host apicomplexan parasite.BMC Genomics. 2014 Aug 20;15(1):696. doi: 10.1186/1471-2164-15-696. BMC Genomics. 2014. PMID: 25142335 Free PMC article.
-
Cell: sporozoite interactions and invasion by apicomplexan parasites of the genus Eimeria.Int J Parasitol. 2001 Jan;31(1):1-8. doi: 10.1016/s0020-7519(00)00150-8. Int J Parasitol. 2001. PMID: 11286188 Review.
-
Perils and Promises of Pathogenic Protozoan Extracellular Vesicles.Front Cell Infect Microbiol. 2020 Aug 14;10:371. doi: 10.3389/fcimb.2020.00371. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32923407 Free PMC article. Review.
Cited by
-
Host innate immune systems gather intel on invading microbes via pathogen-derived extracellular vesicles.Extracell Vesicle. 2024 Jun;3:100043. doi: 10.1016/j.vesic.2024.100043. Epub 2024 May 17. Extracell Vesicle. 2024. PMID: 38939756 Free PMC article.
-
Leishmania Vesicle-Depleted Exoproteome: What, Why, and How?Microorganisms. 2022 Dec 8;10(12):2435. doi: 10.3390/microorganisms10122435. Microorganisms. 2022. PMID: 36557688 Free PMC article. Review.
References
-
- Marcilla A, Trelis M, Cortés A, Sotillo J, Cantalapiedra F, Minguez MT, et al. Extracellular vesicles from parasitic helminths contain specific excretory/secretory proteins and are internalized in intestinal host cells. PLoS ONE. 2012;7:e45974. doi: 10.1371/journal.pone.0045974. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

