Targeted therapy of pyrrolo[2,3-d]pyrimidine antifolates in a syngeneic mouse model of high grade serous ovarian cancer and the impact on the tumor microenvironment
- PMID: 35790779
- PMCID: PMC9256750
- DOI: 10.1038/s41598-022-14788-5
Targeted therapy of pyrrolo[2,3-d]pyrimidine antifolates in a syngeneic mouse model of high grade serous ovarian cancer and the impact on the tumor microenvironment
Abstract
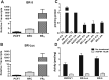
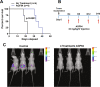
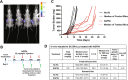
Novel therapies are urgently needed for epithelial ovarian cancer (EOC), the most lethal gynecologic malignancy. In addition, therapies that target unique vulnerabilities in the tumor microenvironment (TME) of EOC have largely been unrealized. One strategy to achieve selective drug delivery for EOC therapy involves use of targeted antifolates via their uptake by folate receptor (FR) proteins, resulting in inhibition of essential one-carbon (C1) metabolic pathways. FRα is highly expressed in EOCs, along with the proton-coupled folate transporter (PCFT); FRβ is expressed on activated macrophages, a major infiltrating immune population in EOC. Thus, there is great potential for targeting both the tumor and the TME with agents delivered via selective transport by FRs and PCFT. In this report, we investigated the therapeutic potential of a novel cytosolic C1 6-substituted pyrrolo[2,3-d]pyrimidine inhibitor AGF94, with selectivity for uptake by FRs and PCFT and inhibition of de novo purine nucleotide biosynthesis, against a syngeneic model of ovarian cancer (BR-Luc) which recapitulates high-grade serous ovarian cancer in patients. In vitro activity of AGF94 was extended in vivo against orthotopic BR-Luc tumors. With late-stage subcutaneous BR-Luc xenografts, AGF94 treatment resulted in substantial anti-tumor efficacy, accompanied by significantly decreased M2-like FRβ-expressing macrophages and increased CD3+ T cells, whereas CD4+ and CD8+ T cells were unaffected. Our studies demonstrate potent anti-tumor efficacy of AGF94 in the therapy of EOC in the context of an intact immune system, and provide a framework for targeting the immunosuppressive TME as an essential component of therapy.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Dual Targeting of Epithelial Ovarian Cancer Via Folate Receptor α and the Proton-Coupled Folate Transporter with 6-Substituted Pyrrolo[2,3-d]pyrimidine Antifolates.Mol Cancer Ther. 2017 May;16(5):819-830. doi: 10.1158/1535-7163.MCT-16-0444. Epub 2017 Jan 30. Mol Cancer Ther. 2017. PMID: 28138029 Free PMC article.
-
Targeting Nonsquamous Nonsmall Cell Lung Cancer via the Proton-Coupled Folate Transporter with 6-Substituted Pyrrolo[2,3-d]Pyrimidine Thienoyl Antifolates.Mol Pharmacol. 2016 Apr;89(4):425-34. doi: 10.1124/mol.115.102798. Epub 2016 Feb 2. Mol Pharmacol. 2016. PMID: 26837243 Free PMC article.
-
Folate Transport and One-Carbon Metabolism in Targeted Therapies of Epithelial Ovarian Cancer.Cancers (Basel). 2021 Dec 31;14(1):191. doi: 10.3390/cancers14010191. Cancers (Basel). 2021. PMID: 35008360 Free PMC article. Review.
-
Tumor Targeting with Novel Pyridyl 6-Substituted Pyrrolo[2,3- d]Pyrimidine Antifolates via Cellular Uptake by Folate Receptor α and the Proton-Coupled Folate Transporter and Inhibition of De Novo Purine Nucleotide Biosynthesis.J Med Chem. 2018 Mar 8;61(5):2027-2040. doi: 10.1021/acs.jmedchem.7b01708. Epub 2018 Feb 21. J Med Chem. 2018. PMID: 29425443 Free PMC article.
-
The human proton-coupled folate transporter: Biology and therapeutic applications to cancer.Cancer Biol Ther. 2012 Dec;13(14):1355-73. doi: 10.4161/cbt.22020. Epub 2012 Sep 6. Cancer Biol Ther. 2012. PMID: 22954694 Free PMC article. Review.
Cited by
-
Biology and therapeutic applications of the proton-coupled folate transporter.Expert Opin Drug Metab Toxicol. 2022 Oct;18(10):695-706. doi: 10.1080/17425255.2022.2136071. Epub 2022 Oct 20. Expert Opin Drug Metab Toxicol. 2022. PMID: 36239195 Free PMC article.
-
Mitochondrial and Cytosolic One-Carbon Metabolism Is a Targetable Metabolic Vulnerability in Cisplatin-Resistant Ovarian Cancer.Mol Cancer Ther. 2024 Jun 4;23(6):809-822. doi: 10.1158/1535-7163.MCT-23-0550. Mol Cancer Ther. 2024. PMID: 38377173 Free PMC article.
-
Recent Advances in Folates and Autoantibodies against Folate Receptors in Early Pregnancy and Miscarriage.Nutrients. 2023 Nov 22;15(23):4882. doi: 10.3390/nu15234882. Nutrients. 2023. PMID: 38068740 Free PMC article. Review.
-
Role of Mitochondrial and Cytosolic Folylpolyglutamate Synthetase in One-Carbon Metabolism and Antitumor Efficacy of Mitochondrial-Targeted Antifolates.Mol Pharmacol. 2024 Sep 17;106(4):173-187. doi: 10.1124/molpharm.124.000912. Mol Pharmacol. 2024. PMID: 39048308
-
Multitargeted 6-Substituted Thieno[2,3-d]pyrimidines as Folate Receptor-Selective Anticancer Agents that Inhibit Cytosolic and Mitochondrial One-Carbon Metabolism.ACS Pharmacol Transl Sci. 2023 Apr 26;6(5):748-770. doi: 10.1021/acsptsci.3c00020. eCollection 2023 May 12. ACS Pharmacol Transl Sci. 2023. PMID: 37200803 Free PMC article.
References
-
- Jackman, A. L., Jansen, G. & Ng, M. in Targeted Drug Strategies for Cancer and Inflammation (eds Jackman, A. L. & Leamon, C. P.) 93–117 (Springer US, 2011).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

