Process Development for Adoptive Cell Therapy in Academia: A Pipeline for Clinical-Scale Manufacturing of Multiple TCR-T Cell Products
- PMID: 35784320
- PMCID: PMC9243500
- DOI: 10.3389/fimmu.2022.896242
Process Development for Adoptive Cell Therapy in Academia: A Pipeline for Clinical-Scale Manufacturing of Multiple TCR-T Cell Products
Abstract
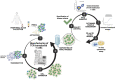
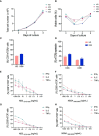
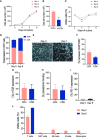
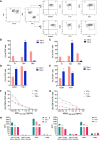
Cellular immunotherapies based on T cell receptor (TCR) transfer are promising approaches for the treatment of cancer and chronic viral infections. The discovery of novel receptors is expanding considerably; however, the clinical development of TCR-T cell therapies still lags. Here we provide a pipeline for process development and clinical-scale manufacturing of TCR-T cells in academia. We utilized two TCRs specific for hepatitis C virus (HCV) as models because of their marked differences in avidity and functional profile in TCR-redirected cells. With our clinical-scale pipeline, we reproduced the functional profile associated with each TCR. Moreover, the two TCR-T cell products demonstrated similar yield, purity, transduction efficiency as well as phenotype. The TCR-T cell products had a highly reproducible yield of over 1.4 × 109 cells, with an average viability of 93%; 97.8-99% of cells were CD3+, of which 47.66 ± 2.02% were CD8+ T cells; the phenotype was markedly associated with central memory (CD62L+CD45RO+) for CD4+ (93.70 ± 5.23%) and CD8+ (94.26 ± 4.04%). The functional assessments in 2D and 3D cell culture assays showed that TCR-T cells mounted a polyfunctional response to the cognate HCV peptide target in tumor cell lines, including killing. Collectively, we report a solid strategy for the efficient large-scale manufacturing of TCR-T cells.
Keywords: T cell modification; TCR-T cells manufacturing; academia; clinical scale; pipeline; process development.
Copyright © 2022 Silva, Chrobok, Rovesti, Healy, Wagner, Maravelia, Gatto, Mazza, Mazzotti, Lohmann, Sällberg Chen, Sällberg, Buggert and Pasetto.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Chronic TCR-MHC (self)-interactions limit the functional potential of TCR affinity-increased CD8 T lymphocytes.J Immunother Cancer. 2019 Nov 5;7(1):284. doi: 10.1186/s40425-019-0773-z. J Immunother Cancer. 2019. PMID: 31690351 Free PMC article.
-
Human effector T cells derived from central memory cells rather than CD8(+)T cells modified by tumor-specific TCR gene transfer possess superior traits for adoptive immunotherapy.Cancer Lett. 2013 Oct 10;339(2):195-207. doi: 10.1016/j.canlet.2013.06.009. Epub 2013 Jun 18. Cancer Lett. 2013. PMID: 23791878
-
Hepatitis C Virus-Specific T Cell Receptor mRNA-Engineered Human T Cells: Impact of Antigen Specificity on Functional Properties.J Virol. 2017 Apr 13;91(9):e00010-17. doi: 10.1128/JVI.00010-17. Print 2017 May 1. J Virol. 2017. PMID: 28228595 Free PMC article.
-
Manufacture of tumor- and virus-specific T lymphocytes for adoptive cell therapies.Cancer Gene Ther. 2015 Mar;22(2):85-94. doi: 10.1038/cgt.2014.81. Epub 2015 Feb 27. Cancer Gene Ther. 2015. PMID: 25721207 Free PMC article. Review.
-
Evolution of CD8+ T Cell Receptor (TCR) Engineered Therapies for the Treatment of Cancer.Cells. 2021 Sep 10;10(9):2379. doi: 10.3390/cells10092379. Cells. 2021. PMID: 34572028 Free PMC article. Review.
Cited by
-
Prognostic significance of peripheral and tumor-infiltrating lymphocytes in newly diagnosed stage III/IV non-small-cell lung cancer.Front Med (Lausanne). 2024 May 22;11:1349178. doi: 10.3389/fmed.2024.1349178. eCollection 2024. Front Med (Lausanne). 2024. PMID: 38841570 Free PMC article.
-
Cellular Cancer Immunotherapy Development and Manufacturing in the Clinic.Clin Cancer Res. 2023 Mar 1;29(5):843-857. doi: 10.1158/1078-0432.CCR-22-2257. Clin Cancer Res. 2023. PMID: 36383184 Free PMC article. Review.
-
Leveraging oncovirus-derived antigen against the viral malignancies in adoptive cell therapies.Biomark Res. 2024 Jul 29;12(1):71. doi: 10.1186/s40364-024-00617-6. Biomark Res. 2024. PMID: 39075601 Free PMC article. Review.
-
T-Cell Receptor Repertoire Sequencing and Its Applications: Focus on Infectious Diseases and Cancer.Int J Mol Sci. 2022 Aug 2;23(15):8590. doi: 10.3390/ijms23158590. Int J Mol Sci. 2022. PMID: 35955721 Free PMC article. Review.
-
ATMP development and pre-GMP environment in academia: a safety net for early cell and gene therapy development and manufacturing.Immunooncol Technol. 2022 Oct 6;16:100099. doi: 10.1016/j.iotech.2022.100099. eCollection 2022 Dec. Immunooncol Technol. 2022. PMID: 36389443 Free PMC article. Review.
References
-
- Castella M, Caballero-Banos M, Ortiz-Maldonado V, Gonzalez-Navarro EA, Sune G, Antonana-Vidosola A, et al. . Point-Of-Care CAR T-Cell Production (ARI-0001) Using a Closed Semi-Automatic Bioreactor: Experience From an Academic Phase I Clinical Trial. Front Immunol (2020) 11:482. doi: 10.3389/fimmu.2020.00482 - DOI - PMC - PubMed
-
- Kochenderfer JN, Dudley ME, Kassim SH, Somerville RP, Carpenter RO, Stetler-Stevenson M, et al. . Chemotherapy-Refractory Diffuse Large B-Cell Lymphoma and Indolent B-Cell Malignancies Can Be Effectively Treated With Autologous T Cells Expressing an Anti-CD19 Chimeric Antigen Receptor. J Clin Oncol (2015) 33(6):540–9. doi: 10.1200/JCO.2014.56.2025 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

