Inconsistencies in Modeling the Efficacy of the Oncolytic Virus HSV1716 Reveal Potential Predictive Biomarkers for Tolerability
- PMID: 35782876
- PMCID: PMC9240779
- DOI: 10.3389/fmolb.2022.889395
Inconsistencies in Modeling the Efficacy of the Oncolytic Virus HSV1716 Reveal Potential Predictive Biomarkers for Tolerability
Abstract
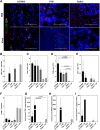
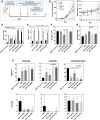
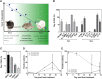
Treatment with HSV1716 via intralesional administration has proven successful for melanoma patients with the hope that oncolytic virotherapy would become another weapon in the systemic anticancer therapy (SACT) arsenal. In addition to challenges surrounding the systemic delivery of oncolytic viruses (OVs), problems associated with its in vivo modeling have resulted in low predictive power, contributing to the observed disappointing clinical efficacy. As OV's efficacy is elicited through interaction with the immune system, syngeneic orthotopic mouse models offer the opportunity to study these with high reproducibility and at a lower cost; however, inbred animals display specific immune characteristics which may confound results. The systemic delivery of HSV1716 was, therefore, assessed in multiple murine models of breast cancer. Tolerability to the virus was strain-dependent with C57/Bl6, the most tolerant and Balb/c experiencing lethal side effects, when delivered intravenously. Maximum tolerated doses were not enough to demonstrate efficacy against tumor growth rates or survival of Balb/c and FVB mouse models; therefore; the most susceptible strain (Balb/c mice) was treated with immunomodulators prior to virus administration in an attempt to reduce side effects. These studies demonstrate the number of variables to consider when modeling the efficacy of OVs and the complexities involved in their interpretation for translational purposes. By reporting these observations, we have potentially revealed a role for T-cell helper polarization in viral tolerability. Importantly, these findings were translated to human studies, whereby a Th1 cytokine profile was expressed in pleural effusions of patients that responded to HSV1716 treatment for malignant pleural mesothelioma with minimal side effects, warranting further investigation as a biomarker for predictive response.
Keywords: T helper cells; biomarker; oncolytic virotherapy; preclinical modeling; tolerability.
Copyright © 2022 Howard, Conner, Danson and Muthana.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Oncolytic herpesvirus therapy for mesothelioma - A phase I/IIa trial of intrapleural administration of HSV1716.Lung Cancer. 2020 Dec;150:145-151. doi: 10.1016/j.lungcan.2020.10.007. Epub 2020 Oct 20. Lung Cancer. 2020. PMID: 33160198 Clinical Trial.
-
Potent efficacy signals from systemically administered oncolytic herpes simplex virus (HSV1716) in hepatocellular carcinoma xenograft models.J Hepatocell Carcinoma. 2014 Oct 16;1:149-61. doi: 10.2147/JHC.S71019. eCollection 2014. J Hepatocell Carcinoma. 2014. PMID: 27508184 Free PMC article.
-
Expression of inhibitor of growth 4 by HSV1716 improves oncolytic potency and enhances efficacy.Cancer Gene Ther. 2012 Jul;19(7):499-507. doi: 10.1038/cgt.2012.24. Epub 2012 May 18. Cancer Gene Ther. 2012. PMID: 22595793
-
Immunovirotherapy: The role of antibody based therapeutics combination with oncolytic viruses.Front Immunol. 2022 Oct 13;13:1012806. doi: 10.3389/fimmu.2022.1012806. eCollection 2022. Front Immunol. 2022. PMID: 36311790 Free PMC article. Review.
-
Immune System, Friend or Foe of Oncolytic Virotherapy?Front Oncol. 2017 May 23;7:106. doi: 10.3389/fonc.2017.00106. eCollection 2017. Front Oncol. 2017. PMID: 28589085 Free PMC article. Review.
Cited by
-
New hopes for the breast cancer treatment: perspectives on the oncolytic virus therapy.Front Immunol. 2024 Mar 21;15:1375433. doi: 10.3389/fimmu.2024.1375433. eCollection 2024. Front Immunol. 2024. PMID: 38576614 Free PMC article. Review.
-
Understanding Immune Responses to Viruses-Do Underlying Th1/Th2 Cell Biases Predict Outcome?Viruses. 2022 Jul 8;14(7):1493. doi: 10.3390/v14071493. Viruses. 2022. PMID: 35891472 Free PMC article. Review.
References
-
- Andtbacka R. H. I., Kaufman H. L., Collichio F., Amatruda T., Senzer N., Chesney J., et al. (2015). Talimogene Laherparepvec Improves Durable Response Rate in Patients with Advanced Melanoma. Jco 33, 2780–2788. 10.1200/jco.2014.58.3377 PubMed Abstract | 10.1200/jco.2014.58.3377 | Google Scholar - DOI - DOI - PubMed
-
- Andtbacka R. H. I., Ross M., Puzanov I., Milhem M., Collichio F., Delman K. A., et al. (2016). Patterns of Clinical Response with Talimogene Laherparepvec (T-VEC) in Patients with Melanoma Treated in the OPTiM Phase III Clinical Trial. Ann. Surg. Oncol. 23, 4169–4177. 10.1245/s10434-016-5286-0 PubMed Abstract | 10.1245/s10434-016-5286-0 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Bareham B., Georgakopoulos N., Matas-Céspedes A., Curran M., Saeb-Parsy K. (2021). Modeling Human Tumor-Immune Environments In Vivo for the Preclinical Assessment of Immunotherapies. Cancer Immunol. Immunother. 70, 2737–2750. 10.1007/s00262-021-02897-5 PubMed Abstract | 10.1007/s00262-021-02897-5 | Google Scholar - DOI - DOI - PMC - PubMed
-
- Belizário J. E. (2009). Immunodeficient Mouse Models: An Overview. Toij 2, 79–85. 10.2174/1874226200902010079 10.2174/1874226200902010079 | Google Scholar - DOI - DOI
-
- Bonaventura P., Shekarian T., Alcazer V., Valladeau-Guilemond J., Valsesia-Wittmann S., Amigorena S., et al. (2019). Cold Tumors: A Therapeutic Challenge for Immunotherapy. Front. Immunol. 10, 168. 10.3389/fimmu.2019.00168 PubMed Abstract | 10.3389/fimmu.2019.00168 | Google Scholar - DOI - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

