Mechanism of opioid addiction and its intervention therapy: Focusing on the reward circuitry and mu-opioid receptor
- PMID: 35774845
- PMCID: PMC9218544
- DOI: 10.1002/mco2.148
Mechanism of opioid addiction and its intervention therapy: Focusing on the reward circuitry and mu-opioid receptor
Abstract
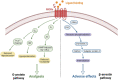
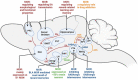
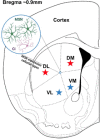
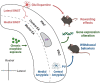
Opioid abuse and addiction have become a global pandemic, posing tremendous health and social burdens. The rewarding effects and the occurrence of withdrawal symptoms are the two mainstays of opioid addiction. Mu-opioid receptors (MORs), a member of opioid receptors, play important roles in opioid addiction, mediating both the rewarding effects of opioids and opioid withdrawal syndrome (OWS). The underlying mechanism of MOR-mediated opioid rewarding effects and withdrawal syndrome is of vital importance to understand the nature of opioid addiction and also provides theoretical basis for targeting MORs to treat drug addiction. In this review, we first briefly introduce the basic concepts of MORs, including their structure, distribution in the nervous system, endogenous ligands, and functional characteristics. We focused on the brain circuitry and molecular mechanism of MORs-mediated opioid reward and withdrawal. The neuroanatomical and functional elements of the neural circuitry of the reward system underlying opioid addiction were thoroughly discussed, and the roles of MOR within the reward circuitry were also elaborated. Furthermore, we interrogated the roles of MORs in OWS, along with the structural basis and molecular adaptions of MORs-mediated withdrawal syndrome. Finally, current treatment strategies for opioid addiction targeting MORs were also presented.
Keywords: dependence; mu‐opioid receptor; opioid addiction; reward circuitry; withdrawal syndrome.
© 2022 The Authors. MedComm published by Sichuan International Medical Exchange & Promotion Association (SCIMEA) and John Wiley & Sons Australia, Ltd.
Conflict of interest statement
The authors declare that there is no conflict of interest.
Figures
Similar articles
-
Endogenous opioid system: a promising target for future smoking cessation medications.Psychopharmacology (Berl). 2017 May;234(9-10):1371-1394. doi: 10.1007/s00213-017-4582-0. Epub 2017 Mar 11. Psychopharmacology (Berl). 2017. PMID: 28285326 Review.
-
Synaptic Regulation by OPRM1 Variants in Reward Neurocircuitry.J Neurosci. 2019 Jul 17;39(29):5685-5696. doi: 10.1523/JNEUROSCI.2317-18.2019. Epub 2019 May 20. J Neurosci. 2019. PMID: 31109961 Free PMC article.
-
Mu Opioid Receptor-Expressing Neurons in the Dorsal Raphe Nucleus Are Involved in Reward Processing and Affective Behaviors.Biol Psychiatry. 2023 Dec 1;94(11):842-851. doi: 10.1016/j.biopsych.2023.05.019. Epub 2023 Jun 5. Biol Psychiatry. 2023. PMID: 37285896 Free PMC article.
-
Mu-Opioid Receptors Expressed in Glutamatergic Neurons are Essential for Morphine Withdrawal.Neurosci Bull. 2020 Oct;36(10):1095-1106. doi: 10.1007/s12264-020-00515-5. Epub 2020 May 25. Neurosci Bull. 2020. PMID: 32451910 Free PMC article.
-
Crosstalk between Mu-Opioid receptors and neuroinflammation: Consequences for drug addiction and pain.Neurosci Biobehav Rev. 2023 Feb;145:105011. doi: 10.1016/j.neubiorev.2022.105011. Epub 2022 Dec 21. Neurosci Biobehav Rev. 2023. PMID: 36565942 Review.
Cited by
-
Fentanyl-induced reward seeking is sex and dose dependent and is prevented by D-cysteine ethylester.Front Pharmacol. 2023 Sep 19;14:1241578. doi: 10.3389/fphar.2023.1241578. eCollection 2023. Front Pharmacol. 2023. PMID: 37795030 Free PMC article.
-
Postoperative Multimodal Analgesia Strategy for Enhanced Recovery After Surgery in Elderly Colorectal Cancer Patients.Pain Ther. 2024 Aug;13(4):745-766. doi: 10.1007/s40122-024-00619-0. Epub 2024 Jun 5. Pain Ther. 2024. PMID: 38836984 Free PMC article. Review.
-
Targeted discovery of gut microbiome-remodeling compounds for the treatment of systemic inflammatory response syndrome.mSystems. 2024 Oct 22;9(10):e0078824. doi: 10.1128/msystems.00788-24. Epub 2024 Sep 5. mSystems. 2024. PMID: 39235366 Free PMC article.
-
Teneurin C-terminal associated peptide (TCAP)-1 attenuates the development and expression of naloxone-precipitated morphine withdrawal in male Swiss Webster mice.Psychopharmacology (Berl). 2024 Aug;241(8):1565-1575. doi: 10.1007/s00213-024-06582-0. Epub 2024 Apr 17. Psychopharmacology (Berl). 2024. PMID: 38630316 Free PMC article.
-
Monoclonal antibody targeting mu-opioid receptor attenuates morphine tolerance via enhancing morphine-induced receptor endocytosis.J Pharm Anal. 2023 Oct;13(10):1135-1152. doi: 10.1016/j.jpha.2023.06.008. Epub 2023 Jun 20. J Pharm Anal. 2023. PMID: 38024852 Free PMC article.
References
-
- McQuay H. Opioids in pain management. Lancet. 1999;353(9171):2229‐2232. - PubMed
-
- Carr DB, Goudas LC. Acute pain. Lancet. 1999;353(9169):2051‐2058. - PubMed
-
- Krauss BS, Calligaris L, Green SM, Barbi E. Current concepts in management of pain in children in the emergency department. Lancet. 2016;387(10013):83‐92. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
