Opposing actions of CRF-R1 and CB1 receptor on facial stimulation-induced MLI-PC plasticity in mouse cerebellar cortex
- PMID: 35754033
- PMCID: PMC9235104
- DOI: 10.1186/s12868-022-00726-8
Opposing actions of CRF-R1 and CB1 receptor on facial stimulation-induced MLI-PC plasticity in mouse cerebellar cortex
Abstract
Background: Corticotropin-releasing factor (CRF) is the major neuromodulator orchestrating the stress response, and is secreted by neurons in various regions of the brain. Cerebellar CRF is released by afferents from inferior olivary neurons and other brainstem nuclei in response to stressful challenges, and contributes to modulation of synaptic plasticity and motor learning behavior via its receptors. We recently found that CRF modulates facial stimulation-evoked molecular layer interneuron-Purkinje cell (MLI-PC) synaptic transmission via CRF type 1 receptor (CRF-R1) in vivo in mice, suggesting that CRF modulates sensory stimulation-evoked MLI-PC synaptic plasticity. However, the mechanism of how CRF modulates MLI-PC synaptic plasticity is unclear. We investigated the effect of CRF on facial stimulation-evoked MLI-PC long-term depression (LTD) in urethane-anesthetized mice by cell-attached recording technique and pharmacological methods.
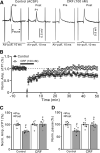
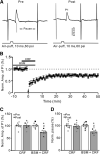
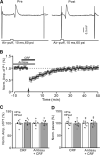
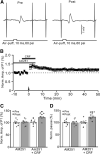
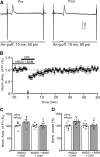
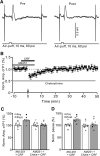
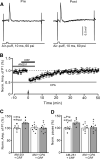
Results: Facial stimulation at 1 Hz induced LTD of MLI-PC synaptic transmission under control conditions, but not in the presence of CRF (100 nM). The CRF-abolished MLI-PC LTD was restored by application of a selective CRF-R1 antagonist, BMS-763,534 (200 nM), but it was not restored by application of a selective CRF-R2 antagonist, antisauvagine-30 (200 nM). Blocking cannabinoid type 1 (CB1) receptor abolished the facial stimulation-induced MLI-PC LTD, and revealed a CRF-triggered MLI-PC long-term potentiation (LTP) via CRF-R1. Notably, either inhibition of protein kinase C (PKC) with chelerythrine (5 µM) or depletion of intracellular Ca2+ with cyclopiazonic acid (100 µM), completely prevented CRF-triggered MLI-PC LTP in mouse cerebellar cortex in vivo.
Conclusions: The present results indicated that CRF blocked sensory stimulation-induced opioid-dependent MLI-PC LTD by triggering MLI-PC LTP through CRF-R1/PKC and intracellular Ca2+ signaling pathway in mouse cerebellar cortex. These results suggest that activation of CRF-R1 opposes opioid-mediated cerebellar MLI-PC plasticity in vivo in mice.
Keywords: Corticotropin-releasing factor (CRF); Long-term plasticity; Molecular layer interneuron (MLI); Mouse cerebellar cortex; Purkinje cell; Sensory stimulation; in vivo cell-attached recording.
© 2022. The Author(s).
Conflict of interest statement
We have no conflicts of interest in this manuscript.
Figures
Similar articles
-
Corticotrophin-Releasing Factor Modulates the Facial Stimulation-Evoked Molecular Layer Interneuron-Purkinje Cell Synaptic Transmission in vivo in Mice.Front Cell Neurosci. 2020 Nov 26;14:563428. doi: 10.3389/fncel.2020.563428. eCollection 2020. Front Cell Neurosci. 2020. PMID: 33324165 Free PMC article.
-
Facial stimulation induces long-term depression at cerebellar molecular layer interneuron-Purkinje cell synapses in vivo in mice.Front Cell Neurosci. 2015 Jun 9;9:214. doi: 10.3389/fncel.2015.00214. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26106296 Free PMC article.
-
Chronic Ethanol Consumption Impairs the Tactile-Evoked Long-Term Depression at Cerebellar Molecular Layer Interneuron-Purkinje Cell Synapses in vivo in Mice.Front Cell Neurosci. 2019 Jan 14;12:521. doi: 10.3389/fncel.2018.00521. eCollection 2018. Front Cell Neurosci. 2019. PMID: 30692916 Free PMC article.
-
Retrograde endocannabinoid signaling in the cerebellar cortex.Cerebellum. 2006;5(2):134-45. doi: 10.1080/14734220600791477. Cerebellum. 2006. PMID: 16818388 Review.
-
Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses.Neuron. 2006 Oct 19;52(2):227-38. doi: 10.1016/j.neuron.2006.09.032. Neuron. 2006. PMID: 17046686 Review.
Cited by
-
Neuropeptides and Their Roles in the Cerebellum.Int J Mol Sci. 2024 Feb 16;25(4):2332. doi: 10.3390/ijms25042332. Int J Mol Sci. 2024. PMID: 38397008 Free PMC article. Review.
-
A subpopulation of oxytocin neurons initiate expression of CRF receptor 1 (CRFR1) in females post parturition.Psychoneuroendocrinology. 2022 Nov;145:105918. doi: 10.1016/j.psyneuen.2022.105918. Epub 2022 Sep 7. Psychoneuroendocrinology. 2022. PMID: 36116320 Free PMC article.
References
-
- Palkovits M, Leranth C, Gorcs T, Young WS., 3rd Corticotropin-releasing factor in the olivocerebellar tract of rats: demonstration by light- and electron-microscopic immunohistochemistry and in situ hybridization histochemistry. Proc Natl Acad Sci USA. 1987;84:3911–3915. doi: 10.1073/pnas.84.11.3911. - DOI - PMC - PubMed
-
- Ezra-Nevo G, Prestori F, Locatelli F, Soda T, Ten Brinke MM, Engel M, Boele HJ, Botta L, Leshkowitz D, Ramot A, Tsoory M, Biton IE, Deussing J, D’Angelo E, De Zeeuw CI, Chen A. Cerebellar Learning Properties Are Modulated by the CRF Receptor. J Neurosci. 2018;38:6751–6765. doi: 10.1523/JNEUROSCI.3106-15.2018. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

