The highly expressed calcium-insensitive synaptotagmin-11 and synaptotagmin-13 modulate insulin secretion
- PMID: 35753051
- PMCID: PMC9541707
- DOI: 10.1111/apha.13857
The highly expressed calcium-insensitive synaptotagmin-11 and synaptotagmin-13 modulate insulin secretion
Abstract
Aim: SYT11 and SYT13, two calcium-insensitive synaptotagmins, are downregulated in islets from type 2 diabetic donors, but their function in insulin secretion is unknown. To address this, we investigated the physiological role of these two synaptotagmins in insulin-secreting cells.
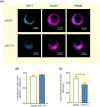
Methods: Correlations between gene expression levels were performed using previously described RNA-seq data on islets from 188 human donors. SiRNA knockdown was performed in EndoC-βH1 and INS-1 832/13 cells. Insulin secretion was measured with ELISA. Patch-clamp was used for single-cell electrophysiology. Confocal microscopy was used to determine intracellular localization.
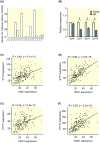
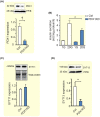
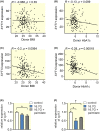
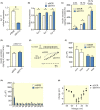
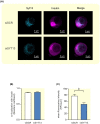
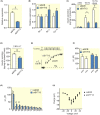
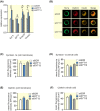
Results: Human islet expression of the transcription factor PDX1 was positively correlated with SYT11 (p = 2.4e-10 ) and SYT13 (p < 2.2e-16 ). Syt11 and Syt13 both co-localized with insulin, indicating their localization in insulin granules. Downregulation of Syt11 in INS-1 832/13 cells (siSYT11) resulted in increased basal and glucose-induced insulin secretion. Downregulation of Syt13 (siSYT13) decreased insulin secretion induced by glucose and K+ . Interestingly, the cAMP-raising agent forskolin was unable to enhance insulin secretion in siSYT13 cells. There was no difference in insulin content, exocytosis, or voltage-gated Ca2+ currents in the two models. Double knockdown of Syt11 and Syt13 (DKD) resembled the results in siSYT13 cells.
Conclusion: SYT11 and SYT13 have similar localization and transcriptional regulation, but they regulate insulin secretion differentially. While downregulation of SYT11 might be a compensatory mechanism in type-2 diabetes, downregulation of SYT13 reduces the insulin secretory response and overrules the compensatory regulation of SYT11 in a way that could aggravate the disease.
Keywords: diabetes; exocytosis; insulin secretion; islet; synaptotagmin; type-2 diabetes.
© 2022 The Authors. Acta Physiologica published by John Wiley & Sons Ltd on behalf of Scandinavian Physiological Society.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
MiR-335 overexpression impairs insulin secretion through defective priming of insulin vesicles.Physiol Rep. 2017 Nov;5(21):e13493. doi: 10.14814/phy2.13493. Physiol Rep. 2017. PMID: 29122960 Free PMC article.
-
Neuronal calcium sensor synaptotagmin-9 is not involved in the regulation of glucose homeostasis or insulin secretion.PLoS One. 2010 Nov 9;5(11):e15414. doi: 10.1371/journal.pone.0015414. PLoS One. 2010. PMID: 21085706 Free PMC article.
-
SUMOylation regulates insulin exocytosis downstream of secretory granule docking in rodents and humans.Diabetes. 2011 Mar;60(3):838-47. doi: 10.2337/db10-0440. Epub 2011 Jan 24. Diabetes. 2011. PMID: 21266332 Free PMC article.
-
Synaptotagmins bind calcium to release insulin.Am J Physiol Endocrinol Metab. 2008 Dec;295(6):E1279-86. doi: 10.1152/ajpendo.90568.2008. Epub 2008 Aug 19. Am J Physiol Endocrinol Metab. 2008. PMID: 18713958 Review.
-
Local and regional control of calcium dynamics in the pancreatic islet.Diabetes Obes Metab. 2017 Sep;19 Suppl 1:30-41. doi: 10.1111/dom.12990. Diabetes Obes Metab. 2017. PMID: 28466490 Review.
Cited by
-
Type 2 diabetes candidate genes, including PAX5, cause impaired insulin secretion in human pancreatic islets.J Clin Invest. 2023 Feb 15;133(4):e163612. doi: 10.1172/JCI163612. J Clin Invest. 2023. PMID: 36656641 Free PMC article.
-
Development of Type 1 Diabetes may occur through a Type 2 Diabetes mechanism.Front Endocrinol (Lausanne). 2022 Dec 14;13:1032822. doi: 10.3389/fendo.2022.1032822. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 36589856 Free PMC article.
-
MicroRNA-218 instructs proper assembly of hippocampal networks.Elife. 2023 Oct 20;12:e82729. doi: 10.7554/eLife.82729. Elife. 2023. PMID: 37862092 Free PMC article.
-
Gene Expression Analysis before and after the Pelvic Flexure in the Epithelium of the Equine Hindgut.Animals (Basel). 2024 Aug 8;14(16):2303. doi: 10.3390/ani14162303. Animals (Basel). 2024. PMID: 39199837 Free PMC article.
References
-
- Wolfes AC, Dean C. The diversity of synaptotagmin isoforms. Curr Opin Neurobiol. 2020;63:198‐209. - PubMed
-
- Iezzi M, Eliasson L, Fukuda M, Wollheim CB. Adenovirus‐mediated silencing of synaptotagmin 9 inhibits Ca2+‐dependent insulin secretion in islets. FEBS Lett. 2005;579(23):5241‐5246. - PubMed
-
- Gauthier BR, Wollheim CB. Synaptotagmins bind calcium to release insulin. Am J Physiol Endocrinol Metab. 2008;295(6):E1279‐E1286. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

