Harnessing anti-cytomegalovirus immunity for local immunotherapy against solid tumors
- PMID: 35749366
- PMCID: PMC9245622
- DOI: 10.1073/pnas.2116738119
Harnessing anti-cytomegalovirus immunity for local immunotherapy against solid tumors
Abstract
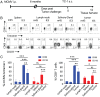
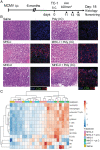
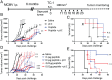
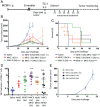
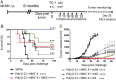
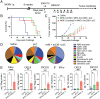
Tumor infiltration by T cells profoundly affects cancer progression and responses to immunotherapy. However, the tumor immunosuppressive microenvironment can impair the induction, trafficking, and local activity of antitumor T cells. Here, we investigated whether intratumoral injection of virus-derived peptide epitopes could activate preexisting antiviral T cell responses locally and promote antitumor responses or antigen spreading. We focused on a mouse model of cytomegalovirus (CMV), a highly prevalent human infection that induces vigorous and durable T cell responses. Mice persistently infected with murine CMV (MCMV) were challenged with lung (TC-1), colon (MC-38), or melanoma (B16-F10) tumor cells. Intratumoral injection of MCMV-derived T cell epitopes triggered in situ and systemic expansion of their cognate, MCMV-specific CD4+ or CD8+ T cells. The MCMV CD8+ T cell epitopes injected alone provoked arrest of tumor growth and some durable remissions. Intratumoral injection of MCMV CD4+ T cell epitopes with polyinosinic acid:polycytidylic acid (pI:C) preferentially elicited tumor antigen-specific CD8+ T cells, promoted tumor clearance, and conferred long-term protection against tumor rechallenge. Notably, secondary proliferation of MCMV-specific CD8+ T cells correlated with better tumor control. Importantly, intratumoral injection of MCMV-derived CD8+ T cell-peptide epitopes alone or CD4+ T cell-peptide epitopes with pI:C induced potent adaptive and innate immune activation of the tumor microenvironment. Thus, CMV-derived peptide epitopes, delivered intratumorally, act as cytotoxic and immunotherapeutic agents to promote immediate tumor control and long-term antitumor immunity that could be used as a stand-alone therapy. The tumor antigen-agnostic nature of this approach makes it applicable across a broad range of solid tumors regardless of their origin.
Keywords: antigen spreading; antiviral immunity; cytomegalovirus; intratumoral immunotherapy; tumor microenvironment.
Conflict of interest statement
Competing interest statement: The authors declare a competing interest. N.C. and J.T.S. have filed an employee invention report through the National Cancer Institute, NIH, related to Novel Cancer Treatment Utilizing Preexisting Microbial Immunity.
Figures


Similar articles
-
A cytomegalovirus-based vaccine expressing a single tumor-specific CD8+ T-cell epitope delays tumor growth in a murine model of prostate cancer.J Immunother. 2012 Jun;35(5):390-9. doi: 10.1097/CJI.0b013e3182585d50. J Immunother. 2012. PMID: 22576344 Free PMC article.
-
Cytomegalovirus-Based Vaccine Expressing a Modified Tumor Antigen Induces Potent Tumor-Specific CD8(+) T-cell Response and Protects Mice from Melanoma.Cancer Immunol Res. 2015 May;3(5):536-46. doi: 10.1158/2326-6066.CIR-14-0044. Epub 2015 Jan 29. Cancer Immunol Res. 2015. PMID: 25633711
-
Therapeutic Vaccination of Hematopoietic Cell Transplantation Recipients Improves Protective CD8 T-Cell Immunotherapy of Cytomegalovirus Infection.Front Immunol. 2021 Aug 19;12:694588. doi: 10.3389/fimmu.2021.694588. eCollection 2021. Front Immunol. 2021. PMID: 34489940 Free PMC article.
-
Intratumoral infection by CMV may change the tumor environment by directly interacting with tumor-associated macrophages to promote cancer immunity.Hum Vaccin Immunother. 2017 Aug 3;13(8):1778-1785. doi: 10.1080/21645515.2017.1331795. Epub 2017 Jun 12. Hum Vaccin Immunother. 2017. PMID: 28604162 Free PMC article. Review.
-
Refining human T-cell immunotherapy of cytomegalovirus disease: a mouse model with 'humanized' antigen presentation as a new preclinical study tool.Med Microbiol Immunol. 2016 Dec;205(6):549-561. doi: 10.1007/s00430-016-0471-0. Epub 2016 Aug 18. Med Microbiol Immunol. 2016. PMID: 27539576 Review.
Cited by
-
QnAs with John T. Schiller.Proc Natl Acad Sci U S A. 2022 Aug 2;119(31):e2209619119. doi: 10.1073/pnas.2209619119. Epub 2022 Jul 18. Proc Natl Acad Sci U S A. 2022. PMID: 35858392 Free PMC article. No abstract available.
-
A prognostic signature based on seven T-cell-related cell clustering genes in bladder urothelial carcinoma.Open Med (Wars). 2023 Sep 18;18(1):20230773. doi: 10.1515/med-2023-0773. eCollection 2023. Open Med (Wars). 2023. PMID: 37745978 Free PMC article.
-
Human Cytomegalovirus Oncoprotection across Diverse Populations, Tumor Histologies, and Age Groups: The Relevance for Prospective Vaccinal Therapy.Int J Mol Sci. 2024 Mar 27;25(7):3741. doi: 10.3390/ijms25073741. Int J Mol Sci. 2024. PMID: 38612552 Free PMC article.
-
Antibody-mediated delivery of viral epitopes to redirect EBV-specific CD8+ T-cell immunity towards cancer cells.Cancer Gene Ther. 2024 Jan;31(1):58-68. doi: 10.1038/s41417-023-00681-4. Epub 2023 Nov 9. Cancer Gene Ther. 2024. PMID: 37945970 Free PMC article.
-
Comparison of methods generating antibody-epitope conjugates for targeting cancer with virus-specific T cells.Front Immunol. 2023 May 16;14:1183914. doi: 10.3389/fimmu.2023.1183914. eCollection 2023. Front Immunol. 2023. PMID: 37261346 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

