Novel Antimicrobial Strategies to Prevent Biofilm Infections in Catheters after Radical Cystectomy: A Pilot Study
- PMID: 35743833
- PMCID: PMC9225455
- DOI: 10.3390/life12060802
Novel Antimicrobial Strategies to Prevent Biofilm Infections in Catheters after Radical Cystectomy: A Pilot Study
Abstract
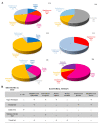
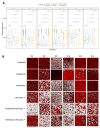
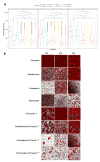
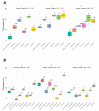
Catheter-associated infections in bladder cancer patients, following radical cystectomy or ureterocutaneostomy, are very frequent, and the development of antibiotic resistance poses great challenges for treating biofilm-based infections. Here, we characterized bacterial communities from catheters of patients who had undergone radical cystectomy for muscle-invasive bladder cancer. We evaluated the efficacy of conventional antibiotics, alone or combined with the human ApoB-derived antimicrobial peptide r(P)ApoBLAla, to treat ureteral catheter-colonizing bacterial communities on clinically isolated bacteria. Microbial communities adhering to indwelling catheters were collected during the patients' regular catheter change schedules (28 days) and extracted within 48 h. Living bacteria were characterized using selective media and biochemical assays. Biofilm growth and novel antimicrobial strategies were analyzed using confocal laser scanning microscopy. Statistical analyses confirmed the relevance of the biofilm reduction induced by conventional antibiotics (fosfomycin, ceftriaxone, ciprofloxacin, gentamicin, and tetracycline) and a well-characterized human antimicrobial peptide r(P)ApoBLAla (1:20 ratio, respectively). Catheters showed polymicrobial communities, with Enterobactericiae and Proteus isolates predominating. In all samples, we recorded a meaningful reduction in biofilms, in both biomass and thickness, upon treatment with the antimicrobial peptide r(P)ApoBLAla in combination with low concentrations of conventional antibiotics. The results suggest that combinations of conventional antibiotics and human antimicrobial peptides might synergistically counteract biofilm growth on ureteral catheters, suggesting novel avenues for preventing catheter-associated infections in patients who have undergone radical cystectomy and ureterocutaneostomy.
Keywords: antibiofilm agents; antimicrobial peptides; antimicrobial resistance; combination therapy; conventional antibiotics; microbial communities; radical cystectomy; urinary catheter-associated infections.
Conflict of interest statement
The authors declare no conflict of interest. The company had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Figures
Similar articles
-
Evaluation of Antimicrobial Durability and Anti-Biofilm Effects in Urinary Catheters Against Enterococcus faecalis Clinical Isolates and Reference Strains.Balkan Med J. 2017 Dec 1;34(6):546-552. doi: 10.4274/balkanmedj.2016.1853. Balkan Med J. 2017. PMID: 29215338 Free PMC article.
-
Catheter-associated urinary tract infections in patients who have undergone radical cystectomy for bladder cancer: A prospective randomized clinical study of two silicone catheters (clinical benefit of antibiotic silicone material).Investig Clin Urol. 2022 May;63(3):334-340. doi: 10.4111/icu.20210436. Epub 2022 Mar 28. Investig Clin Urol. 2022. PMID: 35437959 Free PMC article. Clinical Trial.
-
ELECTRON MICROSCOPIC ASSAY OF BACTERIAL BIOFILM FORMED ON INDWELLING URETHRAL CATHETERS.J Egypt Soc Parasitol. 2016 Dec;46(3):475-484. J Egypt Soc Parasitol. 2016. PMID: 30230743
-
A Glance at Antimicrobial Strategies to Prevent Catheter-Associated Medical Infections.ACS Infect Dis. 2020 Dec 11;6(12):3109-3130. doi: 10.1021/acsinfecdis.0c00526. Epub 2020 Nov 27. ACS Infect Dis. 2020. PMID: 33245664 Review.
-
Biomodification Strategies for the Development of Antimicrobial Urinary Catheters: Overview and Advances.Glob Chall. 2017 Dec 27;2(1):1700068. doi: 10.1002/gch2.201700068. eCollection 2018 Jan 22. Glob Chall. 2017. PMID: 31565299 Free PMC article. Review.
Cited by
-
Laparoscopic Radical Cystectomy with Ileal Orthotopic Neobladder for Bladder Cancer: Current Indications and Outcomes.Urol Int. 2024;108(3):242-253. doi: 10.1159/000535032. Epub 2023 Nov 27. Urol Int. 2024. PMID: 37995673 Free PMC article. Review.
References
-
- Rouprêt M., Babjuk M., Burger M., Capoun O., Cohen D., Compérat E.M., Cowan N.C., Dominguez-Escrig J.L., Gontero P., Hugh Mostafid A., et al. European Association of Urology Guidelines on Upper Urinary Tract Urothelial Carcinoma: 2020 Update. Eur. Urol. 2020;79:62–79. doi: 10.1016/j.eururo.2020.05.042. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

