Selective Upregulation of SIRT1 Expression in Retinal Ganglion Cells by AAV-Mediated Gene Delivery Increases Neuronal Cell Survival and Alleviates Axon Demyelination Associated with Optic Neuritis
- PMID: 35740955
- PMCID: PMC9221096
- DOI: 10.3390/biom12060830
Selective Upregulation of SIRT1 Expression in Retinal Ganglion Cells by AAV-Mediated Gene Delivery Increases Neuronal Cell Survival and Alleviates Axon Demyelination Associated with Optic Neuritis
Abstract
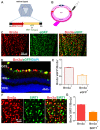
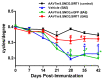
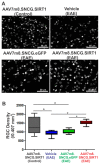
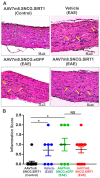
Optic neuritis (ON), the most common ocular manifestation of multiple sclerosis, is an autoimmune inflammatory demyelinating disease also characterized by degeneration of retinal ganglion cells (RGCs) and their axons, which commonly leads to visual impairment despite attempted treatments. Although ON disease etiology is not known, changes in the redox system and exacerbated optic nerve inflammation play a major role in the pathogenesis of the disease. Silent information regulator 1 (sirtuin-1/SIRT1) is a ubiquitously expressed NAD+-dependent deacetylase, which functions to reduce/prevent both oxidative stress and inflammation in various tissues. Non-specific upregulation of SIRT1 by pharmacologic and genetic approaches attenuates RGC loss in experimental ON. Herein, we hypothesized that targeted expression of SIRT1 selectively in RGCs using an adeno-associated virus (AAV) vector as a delivery vehicle is an effective approach to reducing neurodegeneration and preserving vision in ON. We tested this hypothesis through intravitreal injection of AAV7m8.SNCG.SIRT1, an AAV2-derived vector optimized for highly efficient SIRT1 transgene transfer and protein expression into RGCs in mice with experimental autoimmune encephalomyelitis (EAE), a model of multiple sclerosis that recapitulates optic neuritis RGC loss and axon demyelination. Our data show that EAE mice injected with a control vehicle exhibit progressive alteration of visual function reflected by decreasing optokinetic response (OKR) scores, whereas comparatively, AAV7m8.SNCG.SIRT1-injected EAE mice maintain higher OKR scores, suggesting that SIRT1 reduces the visual deficit imparted by EAE. Consistent with this, RGC survival determined by immunolabeling is increased and axon demyelination is decreased in the AAV7m8.SNCG.SIRT1 RGC-injected group of EAE mice compared to the mouse EAE counterpart injected with a vehicle or with control vector AAV7m8.SNCG.eGFP. However, immune cell infiltration of the optic nerve is not significantly different among all EAE groups of mice injected with either vehicle or AAV7m8.SNCG.SIRT1. We conclude that despite minimally affecting the inflammatory response in the optic nerve, AAV7m8-mediated SIRT1 transfer into RGCs has a neuroprotective potential against RGC loss, axon demyelination and vison deficits associated with EAE. Together, these data suggest that SIRT1 exerts direct effects on RGC survival and function.
Keywords: AAV7m8; SIRT1; demyelination; experimental autoimmune encephalomyelitis; gene therapy; inflammation; optic nerve; optic neuritis; retinal ganglion cells.
Conflict of interest statement
A.G.R., K.S.S. and D.S.M. hold intellectual property relevant to this study. A.G.R. and K.S.S. receive research funding from Gyroscope Therapeutics, which was not used in this study. The other authors certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
Figures

Similar articles
-
SIRT1 and NRF2 Gene Transfer Mediate Distinct Neuroprotective Effects Upon Retinal Ganglion Cell Survival and Function in Experimental Optic Neuritis.Invest Ophthalmol Vis Sci. 2018 Mar 1;59(3):1212-1220. doi: 10.1167/iovs.17-22972. Invest Ophthalmol Vis Sci. 2018. PMID: 29494741 Free PMC article.
-
Rescue of retinal ganglion cells in optic nerve injury using cell-selective AAV mediated delivery of SIRT1.Gene Ther. 2021 May;28(5):256-264. doi: 10.1038/s41434-021-00219-z. Epub 2021 Feb 15. Gene Ther. 2021. PMID: 33589779 Free PMC article.
-
Cell-Specific Expression of Human SIRT1 by Gene Therapy Reduces Retinal Ganglion Cell Loss Induced by Elevated Intraocular Pressure.Neurotherapeutics. 2023 Apr;20(3):896-907. doi: 10.1007/s13311-023-01364-6. Epub 2023 Mar 20. Neurotherapeutics. 2023. PMID: 36941497 Free PMC article.
-
Targeting Oxidative Stress for Treatment of Glaucoma and Optic Neuritis.Oxid Med Cell Longev. 2017;2017:2817252. doi: 10.1155/2017/2817252. Epub 2017 Feb 8. Oxid Med Cell Longev. 2017. PMID: 28270908 Free PMC article. Review.
-
Uncovering the Genetics and Physiology behind Optic Neuritis.Genes (Basel). 2023 Dec 9;14(12):2192. doi: 10.3390/genes14122192. Genes (Basel). 2023. PMID: 38137014 Free PMC article. Review.
Cited by
-
Comparison of Brn3a and RBPMS Labeling to Assess Retinal Ganglion Cell Loss During Aging and in a Model of Optic Neuropathy.Invest Ophthalmol Vis Sci. 2024 Apr 1;65(4):19. doi: 10.1167/iovs.65.4.19. Invest Ophthalmol Vis Sci. 2024. PMID: 38587440 Free PMC article.
-
Sirt6 protects retinal ganglion cells and optic nerve from degeneration during aging and glaucoma.Mol Ther. 2024 Jun 5;32(6):1760-1778. doi: 10.1016/j.ymthe.2024.04.030. Epub 2024 Apr 24. Mol Ther. 2024. PMID: 38659223
-
Nanoparticles Enhance Solubility and Neuroprotective Effects of Resveratrol in Demyelinating Disease.Neurotherapeutics. 2023 Jul;20(4):1138-1153. doi: 10.1007/s13311-023-01378-0. Epub 2023 May 9. Neurotherapeutics. 2023. PMID: 37160530 Free PMC article.
-
Treatment with MDL 72527 Ameliorated Clinical Symptoms, Retinal Ganglion Cell Loss, Optic Nerve Inflammation, and Improved Visual Acuity in an Experimental Model of Multiple Sclerosis.Cells. 2022 Dec 16;11(24):4100. doi: 10.3390/cells11244100. Cells. 2022. PMID: 36552864 Free PMC article.
-
Pharmacological Activation and Transgenic Overexpression of SIRT1 Attenuate Traumatic Optic Neuropathy Induced by Blunt Head Impact.Transl Vis Sci Technol. 2024 Sep 3;13(9):27. doi: 10.1167/tvst.13.9.27. Transl Vis Sci Technol. 2024. PMID: 39330985 Free PMC article.
References
-
- Filippi M., Preziosa P., Meani A., Ciccarelli O., Mesaros S., Rovira A., Frederiksen J., Enzinger C., Barkhof F., Gasperini C., et al. Prediction of a multiple sclerosis diagnosis in patients with clinically isolated syndrome using the 2016 MAGNIMS and 2010 McDonald criteria: A retrospective study. Lancet Neurol. 2018;17:133–142. doi: 10.1016/S1474-4422(17)30469-6. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

