Orchestrating the Dermal/Epidermal Tissue Ratio during Wound Healing by Controlling the Moisture Content
- PMID: 35740308
- PMCID: PMC9219632
- DOI: 10.3390/biomedicines10061286
Orchestrating the Dermal/Epidermal Tissue Ratio during Wound Healing by Controlling the Moisture Content
Abstract
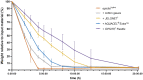
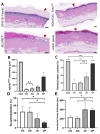
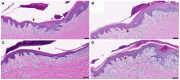
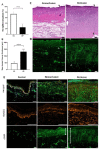
A balanced and moist wound environment and surface increases the effect of various growth factors, cytokines, and chemokines, stimulating cell growth and wound healing. Considering this fact, we tested in vitro and in vivo water evaporation rates from the cellulose dressing epicitehydro when combined with different secondary dressings as well as the resulting wound healing efficacy in a porcine donor site model. The aim of this study was to evaluate how the different rates of water evaporation affected wound healing efficacy. To this end, epicitehydro primary dressing, in combination with different secondary dressing materials (cotton gauze, JELONET◊, AQUACEL® Extra ™, and OPSITE◊ Flexifix), was placed on 3 × 3 cm-sized dermatome wounds with a depth of 1.2 mm on the flanks of domestic pigs. The healing process was analyzed histologically and quantified by morphometry. High water evaporation rates by using the correct secondary dressing, such as cotton gauze, favored a better re-epithelialization in comparison with the low water evaporation resulting from an occlusive secondary dressing, which favored the formation of a new and intact dermal tissue that nearly fully replaced all the dermis that was removed during wounding. This newly available evidence may be of great benefit to clinical wound management.
Keywords: bacterial cellulose dressing; in vivo experiments; moisture balance; secondary wound dressing; wound healing.
Conflict of interest statement
This manuscript has not been published and is not being considered for publication elsewhere in whole or in part in any language. The study was funded by Evomedis GmbH, Austria, of which the author Martin Funk is a part. This had no influence on the collection, analyses, interpretation, or submission of the manuscript. On behalf of all authors, Alexandru-Cristian Tuca declares that the other authors have no conflicts of interest and nothing to disclose.
Figures
Similar articles
-
Management of superficial to partial-thickness wounds.J Athl Train. 2007 Jul-Sep;42(3):422-4. J Athl Train. 2007. PMID: 18059999 Free PMC article.
-
Quality improvement evaluation of postoperative wound dressings in orthopaedic patients.Int J Orthop Trauma Nurs. 2022 May;45:100922. doi: 10.1016/j.ijotn.2022.100922. Epub 2022 Jan 22. Int J Orthop Trauma Nurs. 2022. PMID: 35227950 Review.
-
Comparison of wound healing and patient comfort in partial-thickness burn wounds treated with SUPRATHEL and epictehydro wound dressings.Int Wound J. 2022 May;19(4):782-790. doi: 10.1111/iwj.13674. Epub 2021 Aug 13. Int Wound J. 2022. PMID: 34390204 Free PMC article.
-
Split-thickness skin graft donor site management. A randomized prospective trial comparing a hydrophilic polyurethane absorbent foam dressing with a petrolatum gauze dressing.Arch Otolaryngol Head Neck Surg. 1995 Oct;121(10):1145-9. doi: 10.1001/archotol.1995.01890100055009. Arch Otolaryngol Head Neck Surg. 1995. PMID: 7546582 Clinical Trial.
-
HydroTac-a hydro-responsive wound dressing: a review of the in vitro evidence.J Wound Care. 2022 Jul 2;31(7):540-547. doi: 10.12968/jowc.2022.31.7.540. J Wound Care. 2022. PMID: 35797259 Review.
Cited by
-
Sodium alginate/carboxymethylcellulose gel formulations containing Capparis sepieria plant extract for wound healing.Ther Deliv. 2024;15(12):921-937. doi: 10.1080/20415990.2024.2418800. Epub 2024 Nov 12. Ther Deliv. 2024. PMID: 39529611
-
The Impact of Antiseptic-Loaded Bacterial Nanocellulose on Different Biofilms-An Effective Treatment for Chronic Wounds?J Clin Med. 2022 Nov 9;11(22):6634. doi: 10.3390/jcm11226634. J Clin Med. 2022. PMID: 36431111 Free PMC article.
-
A Highly Standardized Pre-Clinical Porcine Wound Healing Model Powered by Semi-Automated Histological Analysis.Biomedicines. 2024 Jul 31;12(8):1697. doi: 10.3390/biomedicines12081697. Biomedicines. 2024. PMID: 39200162 Free PMC article.
-
A Standardized Porcine Model for Partial-Thickness Wound Healing Studies: Design, Characterization, Model Validation, and Histological Insights.Int J Mol Sci. 2024 Jul 12;25(14):7658. doi: 10.3390/ijms25147658. Int J Mol Sci. 2024. PMID: 39062901 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

