Aberrant activation of p53/p66Shc-mInsc axis increases asymmetric divisions and attenuates proliferation of aged mammary stem cells
- PMID: 35739253
- PMCID: PMC9751089
- DOI: 10.1038/s41418-022-01029-5
Aberrant activation of p53/p66Shc-mInsc axis increases asymmetric divisions and attenuates proliferation of aged mammary stem cells
Abstract
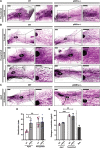
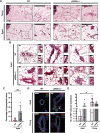
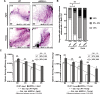
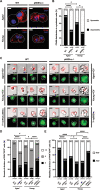
Aging is accompanied by the progressive decline in tissue regenerative capacity and functions of resident stem cells (SCs). Underlying mechanisms, however, remain unclear. Here we show that, during chronological aging, self-renewing mitoses of mammary SCs (MaSCs) are preferentially asymmetric and that their progeny divides less frequently, leading to decreased number of MaSCs and reduced regenerative potential. Underlying mechanisms are investigated in the p66Shc-/- mouse, which exhibits several features of delayed aging, including reduced involution of the mammary gland (MG). p66Shc is a mitochondrial redox sensor that activates a specific p53 transcriptional program, in which the aging-associated p44 isoform of p53 plays a pivotal role. We report here that aged p66Shc-/- MaSCs show increased symmetric divisions, increased proliferation and increased regenerative potential, to an extent reminiscent of young wild-type (WT) MaSCs. Mechanistically, we demonstrate that p66Shc, together with p53: (i) accumulates in the aged MG, (ii) sustains expression of the cell polarity determinant mInscuteable and, concomitantly, (iii) down-regulates critical cell cycle genes (e.g.,: Cdk1 and Cyclin A). Accordingly, overexpression of p53/p44 increases asymmetric divisions and decreases proliferation of young WT MaSCs in a p66Shc-dependent manner and overexpression of mInsc restores WT-like levels of asymmetric divisions in aged p66Shc-/- MaSCs. Notably, deletion of p66Shc has negligible effects in young MaSCs and MG development. These results demonstrate that MG aging is due to aberrant activation of p66Shc, which induces p53/p44 signaling, leading to failure of symmetric divisions, decreased proliferation and reduced regenerative potential of MaSCs.
© 2022. The Author(s), under exclusive licence to ADMC Associazione Differenziamento e Morte Cellulare.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Gambogic Acid Inhibits Malignant Melanoma Cell Proliferation Through Mitochondrial p66shc/ROS-p53/Bax-Mediated Apoptosis.Cell Physiol Biochem. 2016;38(4):1618-30. doi: 10.1159/000443102. Epub 2016 Apr 28. Cell Physiol Biochem. 2016. PMID: 27119348
-
Cyclophosphamide promotes the proliferation inhibition of mouse ovarian granulosa cells and premature ovarian failure by activating the lncRNA-Meg3-p53-p66Shc pathway.Gene. 2017 Jan 5;596:1-8. doi: 10.1016/j.gene.2016.10.011. Epub 2016 Oct 10. Gene. 2017. PMID: 27729272
-
Arginase-II induces vascular smooth muscle cell senescence and apoptosis through p66Shc and p53 independently of its l-arginine ureahydrolase activity: implications for atherosclerotic plaque vulnerability.J Am Heart Assoc. 2013 Jul 5;2(4):e000096. doi: 10.1161/JAHA.113.000096. J Am Heart Assoc. 2013. PMID: 23832324 Free PMC article.
-
Modelling the p53/p66Shc Aging Pathway in the Shortest Living Vertebrate Nothobranchius Furzeri.Aging Dis. 2015 Mar 10;6(2):95-108. doi: 10.14336/AD.2014.0228. eCollection 2015 Mar. Aging Dis. 2015. PMID: 25821638 Free PMC article. Review.
-
Mammary gland stem cells and their application in breast cancer.Oncotarget. 2017 Feb 7;8(6):10675-10691. doi: 10.18632/oncotarget.12893. Oncotarget. 2017. PMID: 27793013 Free PMC article. Review.
Cited by
-
Alleviating hypoxia to improve cancer immunotherapy.Oncogene. 2023 Dec;42(49):3591-3604. doi: 10.1038/s41388-023-02869-2. Epub 2023 Oct 26. Oncogene. 2023. PMID: 37884747 Review.
-
Macrophages maintain mammary stem cell activity and mammary homeostasis via TNF-α-PI3K-Cdk1/Cyclin B1 axis.NPJ Regen Med. 2023 May 2;8(1):23. doi: 10.1038/s41536-023-00296-1. NPJ Regen Med. 2023. PMID: 37130846 Free PMC article.
-
Molecular profiling of a bladder cancer with very high tumour mutational burden.Cell Death Discov. 2024 Apr 30;10(1):202. doi: 10.1038/s41420-024-01883-x. Cell Death Discov. 2024. PMID: 38688924 Free PMC article.
-
Harnessing metabolism of hepatic macrophages to aid liver regeneration.Cell Death Dis. 2023 Aug 29;14(8):574. doi: 10.1038/s41419-023-06066-7. Cell Death Dis. 2023. PMID: 37644019 Free PMC article. Review.
-
p63: a crucial player in epithelial stemness regulation.Oncogene. 2023 Nov;42(46):3371-3384. doi: 10.1038/s41388-023-02859-4. Epub 2023 Oct 17. Oncogene. 2023. PMID: 37848625 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

