Respiratory Epithelial Cells: More Than Just a Physical Barrier to Fungal Infections
- PMID: 35736031
- PMCID: PMC9225092
- DOI: 10.3390/jof8060548
Respiratory Epithelial Cells: More Than Just a Physical Barrier to Fungal Infections
Abstract
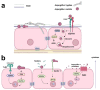
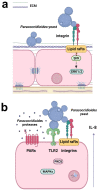
The respiratory epithelium is highly complex, and its composition varies along the conducting airways and alveoli. In addition to their primary function in maintaining the respiratory barrier and lung homeostasis for gas exchange, epithelial cells interact with inhaled pathogens, which can manipulate cell signaling pathways, promoting adhesion to these cells or hosting tissue invasion. Moreover, pathogens (or their products) can induce the secretion of chemokines and cytokines by epithelial cells, and in this way, these host cells communicate with the immune system, modulating host defenses and inflammatory outcomes. This review will focus on the response of respiratory epithelial cells to two human fungal pathogens that cause systemic mycoses: Aspergillus and Paracoccidioides. Some of the host epithelial cell receptors and signaling pathways, in addition to fungal adhesins or other molecules that are responsible for fungal adhesion, invasion, or induction of cytokine secretion will be addressed in this review.
Keywords: Aspergillus; Paracoccidioides; adhesion; airway; cytokine; epithelial cell; invasion; lung; respiratory.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Human Airway Epithelium Responses to Invasive Fungal Infections: A Critical Partner in Innate Immunity.J Fungi (Basel). 2022 Dec 27;9(1):40. doi: 10.3390/jof9010040. J Fungi (Basel). 2022. PMID: 36675861 Free PMC article. Review.
-
The epithelial cell types and their multi-phased defenses against fungi and other pathogens.Clin Chim Acta. 2024 Sep 15;563:119889. doi: 10.1016/j.cca.2024.119889. Epub 2024 Aug 6. Clin Chim Acta. 2024. PMID: 39117034 Review.
-
Pathogenesis II: fungal responses to host responses: interaction of host cells with fungi.Med Mycol. 2000;38 Suppl 1:113-23. Med Mycol. 2000. PMID: 11204137 Review.
-
Aspergillus fumigatus Cell Wall Promotes Apical Airway Epithelial Recruitment of Human Neutrophils.Infect Immun. 2020 Jan 22;88(2):e00813-19. doi: 10.1128/IAI.00813-19. Print 2020 Jan 22. Infect Immun. 2020. PMID: 31767773 Free PMC article.
-
Paracoccidioides species present distinct fungal adherence to epithelial lung cells and promote different IL-8 secretion levels.Med Microbiol Immunol. 2020 Feb;209(1):59-67. doi: 10.1007/s00430-019-00639-0. Epub 2019 Oct 31. Med Microbiol Immunol. 2020. PMID: 31673845
Cited by
-
Paracoccidioides brasiliensis Induces α3 Integrin Lysosomal Degradation in Lung Epithelial Cells.J Fungi (Basel). 2023 Sep 8;9(9):912. doi: 10.3390/jof9090912. J Fungi (Basel). 2023. PMID: 37755020 Free PMC article.
-
Programmed Cell Death in Asthma: Apoptosis, Autophagy, Pyroptosis, Ferroptosis, and Necroptosis.J Inflamm Res. 2023 Jul 1;16:2727-2754. doi: 10.2147/JIR.S417801. eCollection 2023. J Inflamm Res. 2023. PMID: 37415620 Free PMC article. Review.
-
Use of a human small airway epithelial cell line to study the interactions of Aspergillus fumigatus with pulmonary epithelial cells.mSphere. 2023 Oct 24;8(5):e0031423. doi: 10.1128/msphere.00314-23. Epub 2023 Aug 14. mSphere. 2023. PMID: 37578262 Free PMC article.
-
The role of FIBCD1 in response to Aspergillus fumigatus in lung epithelial cells.PLoS One. 2023 Mar 8;18(3):e0282347. doi: 10.1371/journal.pone.0282347. eCollection 2023. PLoS One. 2023. PMID: 36888604 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

