Functional requirements for a Samd14-capping protein complex in stress erythropoiesis
- PMID: 35713400
- PMCID: PMC9282853
- DOI: 10.7554/eLife.76497
Functional requirements for a Samd14-capping protein complex in stress erythropoiesis
Abstract
Acute anemia induces rapid expansion of erythroid precursors and accelerated differentiation to replenish erythrocytes. Paracrine signals-involving cooperation between stem cell factor (SCF)/Kit signaling and other signaling inputs-are required for the increased erythroid precursor activity in anemia. Our prior work revealed that the sterile alpha motif (SAM) domain 14 (Samd14) gene increases the regenerative capacity of the erythroid system in a mouse genetic model and promotes stress-dependent Kit signaling. However, the mechanism underlying Samd14's role in stress erythropoiesis is unknown. We identified a protein-protein interaction between Samd14 and the α- and β-heterodimers of the F-actin capping protein (CP) complex. Knockdown of the CP β subunit increased erythroid maturation in murine ex vivo cultures and decreased colony forming potential of stress erythroid precursors. In a genetic complementation assay for Samd14 activity, our results revealed that the Samd14-CP interaction is a determinant of erythroid precursor cell levels and function. Samd14-CP promotes SCF/Kit signaling in CD71med spleen erythroid precursors. Given the roles of Kit signaling in hematopoiesis and Samd14 in Kit pathway activation, this mechanism may have pathological implications in acute/chronic anemia.
Keywords: Kit; Samd14; capping protein; cell biology; erythropoietin; mouse; regenerative medicine; stem cells; stress erythropoiesis.
Plain language summary
Anemia is a condition in which the body has a shortage of healthy red blood cells to carry enough oxygen to support its organs. A range of factors are known to cause anemia, including traumatic blood loss, toxins or nutritional deficiency. An estimated one-third of all women of reproductive age are anemic, which can cause tiredness, weakness and shortness of breath. Severe anemia drives the release of hormones and growth factors, leading to a rapid regeneration of precursor red blood cells to replenish the supply in the blood. To understand how red blood cell regeneration is controlled, Ray et al. studied proteins involved in regenerating blood using mice in which anemia had been induced with chemicals. Previous research had shown that the protein Samd14 is produced at higher quantities in individuals with anemia, and is involved with the recovery of lost red blood cells. However, it is not known how the Samd14 protein plays a role in regenerating blood cells, or whether Samd14 interacts with other proteins required for red blood cell production. To shed light on these questions, mouse cells exposed to anemia conditions were used to see what proteins Samd14 binds to. Purifying Samd14 revealed that it interacts with the actin capping protein. This interaction relies on a specific region of Samd14 that is similar to regions in other proteins that bind capping proteins. Ray et al. found that the interaction between Samd14 and the actin capping protein increased the signals needed for the development and survival of new red blood cells. These results identify a signaling mechanism that, if disrupted, could cause anemia to develop. They lead to a better understanding of how our bodies recover from anemia, and potential avenues to treat this condition.
© 2022, Ray et al.
Conflict of interest statement
SR, LC, YZ, MS, MN, SA, NW, KH No competing interests declared
Figures

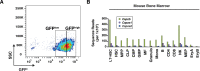





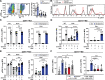
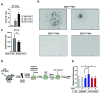
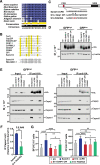
Similar articles
-
Sterile α-motif domain requirement for cellular signaling and survival.J Biol Chem. 2020 May 15;295(20):7113-7125. doi: 10.1074/jbc.RA119.011895. Epub 2020 Apr 2. J Biol Chem. 2020. PMID: 32241909 Free PMC article.
-
GATA Factor-Regulated Samd14 Enhancer Confers Red Blood Cell Regeneration and Survival in Severe Anemia.Dev Cell. 2017 Aug 7;42(3):213-225.e4. doi: 10.1016/j.devcel.2017.07.009. Dev Cell. 2017. PMID: 28787589 Free PMC article.
-
A KIT juxtamembrane PY567 -directed pathway provides nonredundant signals for erythroid progenitor cell development and stress erythropoiesis.Exp Hematol. 2009 Feb;37(2):159-71. doi: 10.1016/j.exphem.2008.10.009. Epub 2008 Dec 18. Exp Hematol. 2009. PMID: 19100679 Free PMC article.
-
Role of c-Kit and erythropoietin receptor in erythropoiesis.Crit Rev Oncol Hematol. 2005 Apr;54(1):63-75. doi: 10.1016/j.critrevonc.2004.11.005. Crit Rev Oncol Hematol. 2005. PMID: 15780908 Review.
-
From stem cell to red cell: regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications.Blood. 2011 Dec 8;118(24):6258-68. doi: 10.1182/blood-2011-07-356006. Epub 2011 Oct 12. Blood. 2011. PMID: 21998215 Free PMC article. Review.
Cited by
-
Sticky, Adaptable, and Many-sided: SAM protein versatility in normal and pathological hematopoietic states.Bioessays. 2023 Aug;45(8):e2300022. doi: 10.1002/bies.202300022. Epub 2023 Jun 15. Bioessays. 2023. PMID: 37318311 Free PMC article. Review.
-
Physiological and regenerative functions of sterile-α motif protein-14 in hematopoiesis.Exp Hematol. 2023 Dec;128:38-47. doi: 10.1016/j.exphem.2023.09.003. Epub 2023 Sep 16. Exp Hematol. 2023. PMID: 37722652 Free PMC article.
References
-
- Agosti V, Corbacioglu S, Ehlers I, Waskow C, Sommer G, Berrozpe G, Kissel H, Tucker CM, Manova K, Moore MAS, Rodewald HR, Besmer P. Critical role for Kit-mediated Src kinase but not PI 3-kinase signaling in pro T and pro B cell development. The Journal of Experimental Medicine. 2004;199:867–878. doi: 10.1084/jem.20031983. - DOI - PMC - PubMed
-
- Agosti V, Karur V, Sathyanarayana P, Besmer P, Wojchowski DM. A KIT juxtamembrane PY567 -directed pathway provides nonredundant signals for erythroid progenitor cell development and stress erythropoiesis. Experimental Hematology. 2009;37:159–171. doi: 10.1016/j.exphem.2008.10.009. - DOI - PMC - PubMed
-
- Astle WJ, Elding H, Jiang T, Allen D, Ruklisa D, Mann AL, Mead D, Bouman H, Riveros-Mckay F, Kostadima MA, Lambourne JJ, Sivapalaratnam S, Downes K, Kundu K, Bomba L, Berentsen K, Bradley JR, Daugherty LC, Delaneau O, Freson K, Garner SF, Grassi L, Guerrero J, Haimel M, Janssen-Megens EM, Kaan A, Kamat M, Kim B, Mandoli A, Marchini J, Martens JHA, Meacham S, Megy K, O’Connell J, Petersen R, Sharifi N, Sheard SM, Staley JR, Tuna S, van der Ent M, Walter K, Wang S-Y, Wheeler E, Wilder SP, Iotchkova V, Moore C, Sambrook J, Stunnenberg HG, Di Angelantonio E, Kaptoge S, Kuijpers TW, Carrillo-de-Santa-Pau E, Juan D, Rico D, Valencia A, Chen L, Ge B, Vasquez L, Kwan T, Garrido-Martín D, Watt S, Yang Y, Guigo R, Beck S, Paul DS, Pastinen T, Bujold D, Bourque G, Frontini M, Danesh J, Roberts DJ, Ouwehand WH, Butterworth AS, Soranzo N. The Allelic Landscape of Human Blood Cell Trait Variation and Links to Common Complex Disease. Cell. 2016;167:1415–1429. doi: 10.1016/j.cell.2016.10.042. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

