Anti-Tumor Effect of IDF-11774, an Inhibitor of Hypoxia-Inducible Factor-1, on Melanoma
- PMID: 35712870
- PMCID: PMC9424330
- DOI: 10.4062/biomolther.2022.061
Anti-Tumor Effect of IDF-11774, an Inhibitor of Hypoxia-Inducible Factor-1, on Melanoma
Abstract
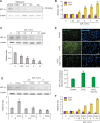
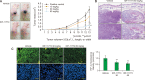
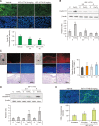
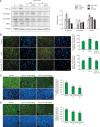
Melanoma is one of the most aggressive skin cancers. Hypoxia contributes to the aggressiveness of melanoma by promoting cancer growth and metastasis. Upregulation of cyclin D1 can promote uncontrolled cell proliferation in melanoma, whereas stimulation of cytotoxic T cell activity can inhibit it. Epithelial mesenchymal transition (EMT) plays a critical role in melanoma metastasis. Hypoxia-inducible factor-1α (HIF-1α) is a main transcriptional mediator that regulates many genes related to hypoxia. CoCl2 is one of the most commonly used hypoxia-mimetic chemicals in cell culture. In this study, inhibitory effects of IDF-11774, an inhibitor of HIF-1α, on melanoma growth and metastasis were examined using cultured B16F10 mouse melanoma cells and nude mice transplanted with B16F10 melanoma cells in the presence or absence of CoCl2-induced hypoxia. IDF-11774 reduced HIF-1α upregulation and cell survival, but increased cytotoxicity of cultured melanoma cells under CoCl2-induced hypoxia. IDF-11774 also reduced tumor size and local invasion of B16F10 melanoma in nude mice along with HIF-1α downregulation. Expression levels of cyclin D1 in melanoma were increased by CoCl2 but decreased by IDF-11774. Apoptosis of melanoma cells and infiltration of cytotoxic T cells were increased in melanoma after treatment with IDF-11774. EMT was stimulated by CoCl2, but restored by IDF- 11774. Overall, IDF-11774 inhibited the growth and metastasis of B16F10 melanoma via HIF-1α downregulation. The growth of B16F10 melanoma was inhibited by cyclin D1 downregulation and cytotoxic T cell stimulation. Metastasis of B16F10 melanoma was inhibited by EMT suppression.
Keywords: HIF-1α inhibitor; IDF11774; Inhibition of melanoma growth and metastasis.
Figures
Similar articles
-
The novel hypoxia-inducible factor-1α inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth.Cell Death Dis. 2017 Jun 1;8(6):e2843. doi: 10.1038/cddis.2017.235. Cell Death Dis. 2017. PMID: 28569777 Free PMC article.
-
STAT3 regulates hypoxia-induced epithelial mesenchymal transition in oesophageal squamous cell cancer.Oncol Rep. 2016 Jul;36(1):108-16. doi: 10.3892/or.2016.4822. Epub 2016 May 19. Oncol Rep. 2016. PMID: 27220595 Free PMC article.
-
HIF-1α promoted vasculogenic mimicry formation in hepatocellular carcinoma through LOXL2 up-regulation in hypoxic tumor microenvironment.J Exp Clin Cancer Res. 2017 Apr 27;36(1):60. doi: 10.1186/s13046-017-0533-1. J Exp Clin Cancer Res. 2017. PMID: 28449718 Free PMC article.
-
Hypoxia Induces Epithelial-Mesenchymal Transition in Follicular Thyroid Cancer: Involvement of Regulation of Twist by Hypoxia Inducible Factor-1α.Yonsei Med J. 2015 Nov;56(6):1503-14. doi: 10.3349/ymj.2015.56.6.1503. Yonsei Med J. 2015. PMID: 26446630 Free PMC article.
-
The role of Hypoxia-Inducible Factor-1alpha and its signaling in melanoma.Biomed Pharmacother. 2021 Sep;141:111873. doi: 10.1016/j.biopha.2021.111873. Epub 2021 Jul 2. Biomed Pharmacother. 2021. PMID: 34225012 Review.
Cited by
-
Immune escape and metastasis mechanisms in melanoma: breaking down the dichotomy.Front Immunol. 2024 Feb 14;15:1336023. doi: 10.3389/fimmu.2024.1336023. eCollection 2024. Front Immunol. 2024. PMID: 38426087 Free PMC article. Review.
-
IDF-11774 Induces Cell Cycle Arrest and Apoptosis by Inhibiting HIF-1α in Gastric Cancer.Pharmaceutics. 2023 Dec 13;15(12):2772. doi: 10.3390/pharmaceutics15122772. Pharmaceutics. 2023. PMID: 38140111 Free PMC article.
-
Novel hypoxia-induced HIF-1αactivation in asthma pathogenesis.Respir Res. 2024 Jul 25;25(1):287. doi: 10.1186/s12931-024-02869-0. Respir Res. 2024. PMID: 39061007 Free PMC article.
References
-
- Ban H. S., Kim B.-K., Lee H., Kim H. M., Harmalkar D., Nam M., Park S.-K., Lee K., Park J.-T., Kim I., Lee K., Hwang G. S., Won M. The novel hypoxia-inducible factor-1α inhibitor IDF-11774 regulates cancer metabolism, thereby suppressing tumor growth. Cell Death Dis. 2017;8:e2843. doi: 10.1038/cddis.2017.235. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

