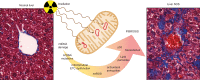
A new mouse model of radiation-induced liver disease reveals mitochondrial dysfunction as an underlying fibrotic stimulus
- PMID: 35712694
- PMCID: PMC9192810
- DOI: 10.1016/j.jhepr.2022.100508
A new mouse model of radiation-induced liver disease reveals mitochondrial dysfunction as an underlying fibrotic stimulus
Abstract
Background & aims: High-dose irradiation is an essential tool to help control the growth of hepatic tumors, but it can cause radiation-induced liver disease (RILD). This life-threatening complication manifests itself months following radiation therapy and is characterized by fibrosis of the pericentral sinusoids. In this study, we aimed to establish a mouse model of RILD to investigate the underlying mechanism of radiation-induced liver fibrosis.
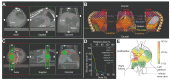
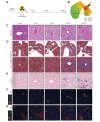
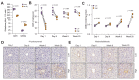
Methods: Using a small animal image-guided radiation therapy platform, an irradiation scheme delivering 50 Gy as a single dose to a focal point in mouse livers was designed. Tissues were analyzed 1 and 6 days, and 6 and 20 weeks post-irradiation. Irradiated livers were assessed by histology, immunohistochemistry, imaging mass cytometry and RNA sequencing. Mitochondrial function was assessed using high-resolution respirometry.
Results: At 6 and 20 weeks post-irradiation, pericentral fibrosis was visible in highly irradiated areas together with immune cell infiltration and extravasation of red blood cells. RNA sequencing analysis showed gene signatures associated with acute DNA damage, p53 activation, senescence and its associated secretory phenotype and fibrosis. Moreover, gene profiles of mitochondrial damage and an increase in mitochondrial DNA heteroplasmy were detected. Respirometry measurements of hepatocytes in vitro confirmed irradiation-induced mitochondrial dysfunction. Finally, the highly irradiated fibrotic areas showed markers of reactive oxygen species such as decreased glutathione and increased lipid peroxides and a senescence-like phenotype.
Conclusions: Based on our mouse model of RILD, we propose that irradiation-induced mitochondrial DNA instability contributes to the development of fibrosis via the generation of excessive reactive oxygen species, p53 pathway activation and a senescence-like phenotype.
Lay summary: Irradiation is an efficient cancer therapy, however, its applicability to the liver is limited by life-threatening radiation-induced hepatic fibrosis. We have developed a new mouse model of radiation-induced liver fibrosis, that recapitulates the human disease. Our model highlights the role of mitochondrial DNA instability in the development of irradiation-induced liver fibrosis. This new model and subsequent findings will help increase our understanding of the hepatic reaction to irradiation and to find strategies that protect the liver, enabling the expanded use of radiotherapy to treat hepatic tumors.
Keywords: 4HNE, 4-hydroxynonenal; CV, central vein; ECM, extracellular matrix; ETC, electron transfer chain; GSH, reduced glutathione (glutathione); GSSG, oxidized glutathione (glutathione disulfide); HSCs, hepatic stellate cells; IGRT, image-guided radiation therapy; IHC, immunohistochemistry; IMC, imaging mass cytometry; MDA, malondialdehyde; RILD, radiation-induced liver disease; RNAseq, RNA sequencing; ROS; ROS, reactive oxygen species; RT, radiation therapy; SASP, senescence-associated secretory phenotype; SNP, single nucleotide polymorphism; SOS, sinusoidal obstruction syndrome; fibrosis; image guided radiation therapy (IGRT); mitochondrial dysfunction; mitochondrial-DNA; mouse model; mtDNA, mitochondrial DNA; mtROS, mitochondrial reactive oxygen species; p53; radiation-induced liver disease (RILD); rcf, relative centrifuge force; senescence; sinusoidal obstruction syndrome.
© 2022 The Authors.
Conflict of interest statement
The authors declare no conflicts of interest that pertain to this work. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


Similar articles
-
Cooperation of liver cells in health and disease.Adv Anat Embryol Cell Biol. 2001;161:III-XIII, 1-151. doi: 10.1007/978-3-642-56553-3. Adv Anat Embryol Cell Biol. 2001. PMID: 11729749 Review.
-
Serine Protease HtrA2/Omi Deficiency Impairs Mitochondrial Homeostasis and Promotes Hepatic Fibrogenesis via Activation of Hepatic Stellate Cells.Cells. 2019 Sep 20;8(10):1119. doi: 10.3390/cells8101119. Cells. 2019. PMID: 31547195 Free PMC article.
-
Irradiation-induced senescence of bone marrow mesenchymal stem cells aggravates osteogenic differentiation dysfunction via paracrine signaling.Am J Physiol Cell Physiol. 2020 May 1;318(5):C1005-C1017. doi: 10.1152/ajpcell.00520.2019. Epub 2020 Apr 1. Am J Physiol Cell Physiol. 2020. PMID: 32233952
-
TERT alleviates irradiation-induced late rectal injury by reducing hypoxia-induced ROS levels through the activation of NF-κB and autophagy.Int J Mol Med. 2016 Sep;38(3):785-93. doi: 10.3892/ijmm.2016.2673. Epub 2016 Jul 11. Int J Mol Med. 2016. PMID: 27431814 Free PMC article.
-
Radiation-induced liver disease: beyond DNA damage.Cell Cycle. 2023 Mar;22(5):506-526. doi: 10.1080/15384101.2022.2131163. Epub 2022 Oct 10. Cell Cycle. 2023. PMID: 36214587 Free PMC article. Review.
Cited by
-
Late Effects of Ionizing Radiation on the Ultrastructure of Hepatocytes and Activity of Lysosomal Enzymes in Mouse Liver Irradiated In Vivo.Metabolites. 2024 Apr 9;14(4):212. doi: 10.3390/metabo14040212. Metabolites. 2024. PMID: 38668340 Free PMC article.
-
Multiplexed Imaging Mass Cytometry Analysis in Preclinical Models of Pancreatic Cancer.Int J Mol Sci. 2024 Jan 23;25(3):1389. doi: 10.3390/ijms25031389. Int J Mol Sci. 2024. PMID: 38338669 Free PMC article.
-
Tissue fibrosis induced by radiotherapy: current understanding of the molecular mechanisms, diagnosis and therapeutic advances.J Transl Med. 2023 Oct 9;21(1):708. doi: 10.1186/s12967-023-04554-0. J Transl Med. 2023. PMID: 37814303 Free PMC article. Review.
-
Current Insights into Molecular Mechanisms and Potential Biomarkers for Treating Radiation-Induced Liver Damage.Cells. 2024 Sep 16;13(18):1560. doi: 10.3390/cells13181560. Cells. 2024. PMID: 39329744 Free PMC article. Review.
-
Single-cell high-dimensional imaging mass cytometry: one step beyond in oncology.Semin Immunopathol. 2023 Jan;45(1):17-28. doi: 10.1007/s00281-022-00978-w. Epub 2023 Jan 4. Semin Immunopathol. 2023. PMID: 36598557 Free PMC article. Review.
References
-
- Mahadevan A., Blanck O., Lanciano R., Peddada A., Sundararaman S., D'Ambrosio D., et al. Stereotactic Body Radiotherapy (SBRT) for liver metastasis - clinical outcomes from the international multi-institutional RSSearch(R) Patient Registry. Radiat Oncol. 2018;13(1):26. doi: 10.1186/s13014-018-0969-2. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

