NLRP1 Inflammasomes: A Potential Target for the Treatment of Several Types of Brain Injury
- PMID: 35707533
- PMCID: PMC9189285
- DOI: 10.3389/fimmu.2022.863774
NLRP1 Inflammasomes: A Potential Target for the Treatment of Several Types of Brain Injury
Abstract
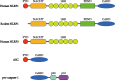
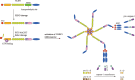
NOD-like receptor (NLR) family pyrin domain-containing 1 (NLRP1) is a member of the NLR family. The NLRP1 inflammasome consists of the NLRP1 protein, the adaptor protein apoptosis-associated speck-like protein containing a CARD domain, and the effector molecule pro-caspase-1. When stimulated, the inflammasome initiates the cleavage of pro-caspase-1 and converts it into its active form, caspase-1; then, caspase-1 facilitates the cleavage of the proinflammatory cytokines interleukin-1β and interleukin-18 into their active and secreted forms. In addition, caspase-1 also mediates the cleavage of gasdermin D, which leads to pyroptosis, an inflammatory form of cell death. Pathological events that damage the brain and result in neuropathological conditions can generally be described as brain injury. Neuroinflammation, especially that driven by NLRP1, plays a considerable role in the pathophysiology of brain injury, such as early brain injury (EBI) of subarachnoid hemorrhage, ischemic brain injury during stroke, and traumatic brain injury (TBI). In this article, a thorough overview of NLRP1 is presented, including its structure, mechanism of activation, and role in neuroinflammation. We also present recent studies on NLRP1 as a target for the treatment of EBI, ischemic brain injury, TBI, and other types of brain injury, thus highlighting the perspective of NLRP1 as an effective mediator of catastrophic brain injury.
Keywords: NLRP1 inflammasome; neuroinflammation; stroke; subarachnoid hemorrhage; traumatic brain injury.
Copyright © 2022 Mi, Min, Chai, Zhang and Chen.
Conflict of interest statement
The authors declare that this research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Cytokine Secretion and Pyroptosis of Thyroid Follicular Cells Mediated by Enhanced NLRP3, NLRP1, NLRC4, and AIM2 Inflammasomes Are Associated With Autoimmune Thyroiditis.Front Immunol. 2018 Jun 4;9:1197. doi: 10.3389/fimmu.2018.01197. eCollection 2018. Front Immunol. 2018. PMID: 29915579 Free PMC article.
-
The pathological role of NLRs and AIM2 inflammasome-mediated pyroptosis in damaged blood-brain barrier after traumatic brain injury.Brain Res. 2018 Oct 15;1697:10-20. doi: 10.1016/j.brainres.2018.06.008. Epub 2018 Jun 8. Brain Res. 2018. PMID: 29886252
-
Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke.Cell Death Dis. 2013 Sep 5;4(9):e790. doi: 10.1038/cddis.2013.326. Cell Death Dis. 2013. PMID: 24008734 Free PMC article.
-
Inflammasome: Its role in traumatic brain and spinal cord injury.J Cell Physiol. 2018 Jul;233(7):5160-5169. doi: 10.1002/jcp.26287. Epub 2018 Jan 19. J Cell Physiol. 2018. PMID: 29150951 Review.
-
The NLRP1 inflammasomes.Immunol Rev. 2015 May;265(1):22-34. doi: 10.1111/imr.12283. Immunol Rev. 2015. PMID: 25879281 Review.
Cited by
-
Nef mediates neuroimmune response, myelin impairment, and neuronal injury in EcoHIV-infected mice.Life Sci Alliance. 2024 Nov 12;8(2):e202402879. doi: 10.26508/lsa.202402879. Print 2025 Feb. Life Sci Alliance. 2024. PMID: 39532531 Free PMC article.
-
The Neglected Sibling: NLRP2 Inflammasome in the Nervous System.Aging Dis. 2024 May 7;15(3):1006-1028. doi: 10.14336/AD.2023.0926-1. Aging Dis. 2024. PMID: 38722788 Free PMC article. Review.
-
Effects of early pregnancy on NOD-like receptor expression in the ovine endometrium.Front Vet Sci. 2024 Jun 5;11:1384386. doi: 10.3389/fvets.2024.1384386. eCollection 2024. Front Vet Sci. 2024. PMID: 38903689 Free PMC article.
-
Detrimental Roles of Hypoxia-Inducible Factor-1α in Severe Hypoxic Brain Diseases.Int J Mol Sci. 2024 Apr 18;25(8):4465. doi: 10.3390/ijms25084465. Int J Mol Sci. 2024. PMID: 38674050 Free PMC article. Review.
-
The interaction of inflammasomes and gut microbiota: novel therapeutic insights.Cell Commun Signal. 2024 Apr 2;22(1):209. doi: 10.1186/s12964-024-01504-1. Cell Commun Signal. 2024. PMID: 38566180 Free PMC article. Review.
References
-
- Fan H, Ding R, Liu W, Zhang X, Li R, Wei B, et al. . Heat Shock Protein 22 Modulates NRF1/TFAM-Dependent Mitochondrial Biogenesis and DRP1-Sparked Mitochondrial Apoptosis Through AMPK-Pgc1α Signaling Pathway to Alleviate the Early Brain Injury of Subarachnoid Hemorrhage in Rats. Redox Biol (2021) 40:101856. doi: 10.1016/j.redox.2021.101856 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

