A guide to membrane atg8ylation and autophagy with reflections on immunity
- PMID: 35699692
- PMCID: PMC9202678
- DOI: 10.1083/jcb.202203083
A guide to membrane atg8ylation and autophagy with reflections on immunity
Abstract
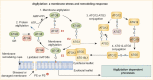
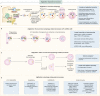
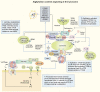
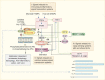
The process of membrane atg8ylation, defined herein as the conjugation of the ATG8 family of ubiquitin-like proteins to membrane lipids, is beginning to be appreciated in its broader manifestations, mechanisms, and functions. Classically, membrane atg8ylation with LC3B, one of six mammalian ATG8 family proteins, has been viewed as the hallmark of canonical autophagy, entailing the formation of characteristic double membranes in the cytoplasm. However, ATG8s are now well described as being conjugated to single membranes and, most recently, proteins. Here we propose that the atg8ylation is coopted by multiple downstream processes, one of which is canonical autophagy. We elaborate on these biological outputs, which impact metabolism, quality control, and immunity, emphasizing the context of inflammation and immunological effects. In conclusion, we propose that atg8ylation is a modification akin to ubiquitylation, and that it is utilized by different systems participating in membrane stress responses and membrane remodeling activities encompassing autophagy and beyond.
© 2022 Deretic and Lazarou.
Figures

Similar articles
-
Conjugation of ATG8s to single membranes at a glance.J Cell Sci. 2024 Aug 1;137(15):jcs261031. doi: 10.1242/jcs.261031. Epub 2024 Aug 15. J Cell Sci. 2024. PMID: 39145464 Free PMC article. Review.
-
Membrane atg8ylation in Canonical and Noncanonical Autophagy.J Mol Biol. 2024 Aug 1;436(15):168532. doi: 10.1016/j.jmb.2024.168532. Epub 2024 Mar 12. J Mol Biol. 2024. PMID: 38479594 Review.
-
ATG8ylation of proteins: A way to cope with cell stress?J Cell Biol. 2021 Nov 1;220(11):e202108120. doi: 10.1083/jcb.202108120. Epub 2021 Oct 20. J Cell Biol. 2021. PMID: 34671813 Free PMC article.
-
Atg8ylation as a general membrane stress and remodeling response.Cell Stress. 2021 Aug 12;5(9):128-142. doi: 10.15698/cst2021.09.255. eCollection 2021 Sep. Cell Stress. 2021. PMID: 34527862 Free PMC article. Review.
-
Non-canonical autophagy drives alternative ATG8 conjugation to phosphatidylserine.Mol Cell. 2021 May 6;81(9):2031-2040.e8. doi: 10.1016/j.molcel.2021.03.020. Epub 2021 Apr 27. Mol Cell. 2021. PMID: 33909989 Free PMC article.
Cited by
-
An expanding repertoire of E3 ligases in membrane Atg8ylation.Nat Cell Biol. 2024 Mar;26(3):307-308. doi: 10.1038/s41556-023-01329-z. Nat Cell Biol. 2024. PMID: 38225349 Free PMC article. No abstract available.
-
LC3-associated phagocytosis promotes glial degradation of axon debris after injury in Drosophila models.Nat Commun. 2023 May 29;14(1):3077. doi: 10.1038/s41467-023-38755-4. Nat Commun. 2023. PMID: 37248218 Free PMC article.
-
Mitophagy in the aging nervous system.Front Cell Dev Biol. 2022 Oct 11;10:978142. doi: 10.3389/fcell.2022.978142. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36303604 Free PMC article. Review.
-
Conjugation of ATG8s to single membranes at a glance.J Cell Sci. 2024 Aug 1;137(15):jcs261031. doi: 10.1242/jcs.261031. Epub 2024 Aug 15. J Cell Sci. 2024. PMID: 39145464 Free PMC article. Review.
-
Atg8ylation as a host-protective mechanism against Mycobacterium tuberculosis.Front Tuberc. 2023;1:1275882. doi: 10.3389/ftubr.2023.1275882. Epub 2023 Sep 29. Front Tuberc. 2023. PMID: 37901138 Free PMC article.

