Evidence of beta amyloid independent small vessel disease in familial Alzheimer's disease
- PMID: 35695802
- PMCID: PMC9616091
- DOI: 10.1111/bpa.13097
Evidence of beta amyloid independent small vessel disease in familial Alzheimer's disease
Abstract
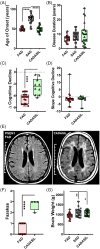
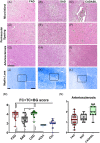
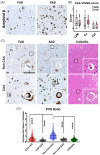
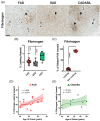
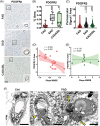
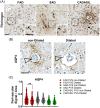
We studied small vessel disease (SVD) pathology in Familial Alzheimer's disease (FAD) subjects carrying the presenilin 1 (PSEN1) p.Glu280Ala mutation in comparison to those with sporadic Alzheimer's disease (SAD) as a positive control for Alzheimer's pathology and Cerebral Autosomal Dominant Arteriopathy with Subcortical Infarcts and Leukoencephalopathy (CADASIL) bearing different NOTCH3 mutations, as positive controls for SVD pathology. Upon magnetic resonance imaging (MRI) in life, some FAD showed mild white matter hyperintensities and no further radiologic evidence of SVD. In post-mortem studies, total SVD pathology in cortical areas and basal ganglia was similar in PSEN1 FAD and CADASIL subjects, except for the feature of arteriosclerosis which was higher in CADASIL subjects than in PSEN1 FAD subjects. Further only a few SAD subjects showed a similar degree of SVD pathology as observed in CADASIL. Furthermore, we found significantly enlarged perivascular spaces in vessels devoid of cerebral amyloid angiopathy in FAD compared with SAD and CADASIL subjects. As expected, there was greater fibrinogen-positive perivascular reactivity in CADASIL but similar reactivity in PSEN1 FAD and SAD groups. Fibrinogen immunoreactivity correlated with onset age in the PSEN1 FAD cases, suggesting increased vascular permeability may contribute to cognitive decline. Additionally, we found reduced perivascular expression of PDGFRβ AQP4 in microvessels with enlarged PVS in PSEN1 FAD cases. We demonstrate that there is Aβ-independent SVD pathology in PSEN1 FAD, that was marginally lower than that in CADASIL subjects although not evident by MRI. These observations suggest presence of covert SVD even in PSEN1, contributing to disease progression. As is the case in SAD, these consequences may be preventable by early recognition and actively controlling vascular disease risk, even in familial forms of dementia.
Keywords: Alzheimer's disease; FAD; cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy; dementia; presenilin; small vessel disease; vascular disease.
© 2022 The Authors. Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Global Cardiovascular Risk Profile and Cerebrovascular Abnormalities in Presymptomatic Individuals with CADASIL or Autosomal Dominant Alzheimer's Disease.J Alzheimers Dis. 2021;82(2):841-853. doi: 10.3233/JAD-210313. J Alzheimers Dis. 2021. PMID: 34092645 Free PMC article.
-
Variability in the type and layer distribution of cortical Aβ pathology in familial Alzheimer's disease.Brain Pathol. 2022 May;32(3):e13009. doi: 10.1111/bpa.13009. Epub 2021 Jul 28. Brain Pathol. 2022. PMID: 34319632 Free PMC article.
-
Neurons derived from sporadic Alzheimer's disease iPSCs reveal elevated TAU hyperphosphorylation, increased amyloid levels, and GSK3B activation.Alzheimers Res Ther. 2017 Dec 1;9(1):90. doi: 10.1186/s13195-017-0317-z. Alzheimers Res Ther. 2017. PMID: 29191219 Free PMC article.
-
New insights into mechanisms of small vessel disease stroke from genetics.Clin Sci (Lond). 2017 Apr 1;131(7):515-531. doi: 10.1042/CS20160825. Clin Sci (Lond). 2017. PMID: 28302914 Review.
-
Evidence For and Against a Pathogenic Role of Reduced γ-Secretase Activity in Familial Alzheimer's Disease.J Alzheimers Dis. 2016 Apr 4;52(3):781-99. doi: 10.3233/JAD-151186. J Alzheimers Dis. 2016. PMID: 27060961 Review.
Cited by
-
Gliovascular alterations in sporadic and familial Alzheimer's disease: APOE3 Christchurch homozygote glioprotection.Brain Pathol. 2023 Mar;33(2):e13119. doi: 10.1111/bpa.13119. Epub 2022 Sep 21. Brain Pathol. 2023. PMID: 36130084 Free PMC article.
-
Advancements in dementia research, diagnostics, and care in Latin America: Highlights from the 2023 Alzheimer's Association International conference satellite symposium in Mexico City.Alzheimers Dement. 2024 Jul;20(7):5009-5026. doi: 10.1002/alz.13850. Epub 2024 May 27. Alzheimers Dement. 2024. PMID: 38801124 Free PMC article. Review.
-
Iron accumulation/overload and Alzheimer's disease risk factors in the precuneus region: A comprehensive narrative review.Aging Med (Milton). 2024 Oct 22;7(5):649-667. doi: 10.1002/agm2.12363. eCollection 2024 Oct. Aging Med (Milton). 2024. PMID: 39507230 Free PMC article. Review.
-
The 2022 symposium on dementia and brain aging in low- and middle-income countries: Highlights on research, diagnosis, care, and impact.Alzheimers Dement. 2024 Jun;20(6):4290-4314. doi: 10.1002/alz.13836. Epub 2024 May 2. Alzheimers Dement. 2024. PMID: 38696263 Free PMC article. Review.
-
Distinct tau neuropathology and cellular profiles of an APOE3 Christchurch homozygote protected against autosomal dominant Alzheimer's dementia.Acta Neuropathol. 2022 Sep;144(3):589-601. doi: 10.1007/s00401-022-02467-8. Epub 2022 Jul 15. Acta Neuropathol. 2022. PMID: 35838824 Free PMC article.
References
-
- Prince M, Ali GC, Guerchet M, Wu YT, Prina M, Wimo A. World Alzheimer Report 2015: The Global Impact of Dementia. 1st ed. London, UK: Alzheimer's Disease International; 2015.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

