Impact of N-Terminal Tags on De Novo Vimentin Intermediate Filament Assembly
- PMID: 35683030
- PMCID: PMC9181571
- DOI: 10.3390/ijms23116349
Impact of N-Terminal Tags on De Novo Vimentin Intermediate Filament Assembly
Abstract
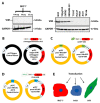
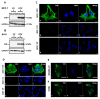
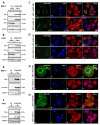
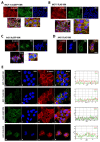
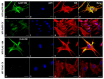
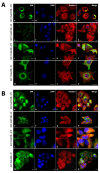
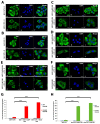
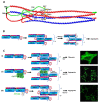
Vimentin, a type III intermediate filament protein, is found in most cells along with microfilaments and microtubules. It has been shown that the head domain folds back to associate with the rod domain and this association is essential for filament assembly. The N-terminally tagged vimentin has been widely used to label the cytoskeleton in live cell imaging. Although there is previous evidence that EGFP tagged vimentin fails to form filaments but is able to integrate into a pre-existing network, no study has systematically investigated or established a molecular basis for this observation. To determine whether a tag would affect de novo filament assembly, we used vimentin fused at the N-terminus with two different sized tags, AcGFP (239 residues, 27 kDa) and 3 × FLAG (22 residues; 2.4 kDa) to assemble into filaments in two vimentin-deficient epithelial cells, MCF-7 and A431. We showed that regardless of tag size, N-terminally tagged vimentin aggregated into globules with a significant proportion co-aligning with β-catenin at cell-cell junctions. However, the tagged vimentin aggregates could form filaments upon adding untagged vimentin at a ratio of 1:1 or when introduced into cells containing pre-existing filaments. The resultant filament network containing a mixture of tagged and untagged vimentin was less stable compared to that formed by only untagged vimentin. The data suggest that placing a tag at the N-terminus may create steric hinderance in case of a large tag (AcGFP) or electrostatic repulsion in case of highly charged tag (3 × FLAG) perhaps inducing a conformational change, which deleteriously affects the association between head and rod domains. Taken together our results shows that a free N-terminus is essential for filament assembly as N-terminally tagged vimentin is not only incapable of forming filaments, but it also destabilises when integrated into a pre-existing network.
Keywords: ectopic protein expression; fusion proteins; immunofluorescence; intermediate filaments; protein domains.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Structural elements of the amino-terminal head domain of vimentin essential for intermediate filament formation in vivo and in vitro.Exp Cell Res. 1994 Jul;213(1):128-42. doi: 10.1006/excr.1994.1182. Exp Cell Res. 1994. PMID: 8020583
-
Assembly of amino-terminally deleted desmin in vimentin-free cells.J Cell Biol. 1990 Nov;111(5 Pt 1):1971-85. doi: 10.1083/jcb.111.5.1971. J Cell Biol. 1990. PMID: 1699950 Free PMC article.
-
Salt-stable interaction of the amino-terminal head region of vimentin with the alpha-helical rod domain of cytoplasmic intermediate filament proteins and its relevance to protofilament structure and filament formation and stability.J Cell Sci. 1992 Feb;101 ( Pt 2):363-81. doi: 10.1242/jcs.101.2.363. J Cell Sci. 1992. PMID: 1629250
-
Intermediate filament proteins are reliable immunohistological biomarkers to help diagnose multiple tissue-specific diseases.Anat Histol Embryol. 2023 Sep;52(5):655-672. doi: 10.1111/ahe.12937. Epub 2023 Jun 16. Anat Histol Embryol. 2023. PMID: 37329162 Review.
-
The unique biomechanics of intermediate filaments - From single filaments to cells and tissues.Curr Opin Cell Biol. 2023 Dec;85:102263. doi: 10.1016/j.ceb.2023.102263. Epub 2023 Oct 21. Curr Opin Cell Biol. 2023. PMID: 37871499 Review.
Cited by
-
Unraveling Desmin's Head Domain Structure and Function.Cells. 2024 Mar 29;13(7):603. doi: 10.3390/cells13070603. Cells. 2024. PMID: 38607042 Free PMC article.
-
Effects of truncations in the N- and C-terminal domains of filensin on filament formation with phakinin in cell-free conditions and cultured cells.FEBS Open Bio. 2023 Nov;13(11):1990-2004. doi: 10.1002/2211-5463.13700. Epub 2023 Aug 30. FEBS Open Bio. 2023. PMID: 37615966 Free PMC article.
-
Transcriptome Analysis Reveals Vimentin-Induced Disruption of Cell-Cell Associations Augments Breast Cancer Cell Migration.Cells. 2022 Dec 13;11(24):4035. doi: 10.3390/cells11244035. Cells. 2022. PMID: 36552797 Free PMC article.
-
Site-Directed Mutagenesis to Mutate Multiple Residues in a Single Reaction.Methods Mol Biol. 2024;2849:123-133. doi: 10.1007/7651_2023_511. Methods Mol Biol. 2024. PMID: 38180689
-
Are the Head and Tail Domains of Intermediate Filaments Really Unstructured Regions?Genes (Basel). 2024 May 16;15(5):633. doi: 10.3390/genes15050633. Genes (Basel). 2024. PMID: 38790262 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

