Pitavastatin Is Anti-Leukemic in a Bone Marrow Microenvironment Model of B-Lineage Acute Lymphoblastic Leukemia
- PMID: 35681662
- PMCID: PMC9179467
- DOI: 10.3390/cancers14112681
Pitavastatin Is Anti-Leukemic in a Bone Marrow Microenvironment Model of B-Lineage Acute Lymphoblastic Leukemia
Abstract
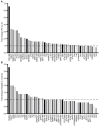
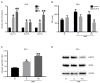
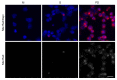
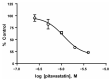
The lack of complete therapeutic success in the treatment of B-cell acute lymphoblastic leukemia (ALL) has been attributed, in part, to a subset of cells within the bone marrow microenvironment that are drug resistant. Recently, the cholesterol synthesis inhibitor, pitavastatin (PIT), was shown to be active in acute myeloid leukemia, prompting us to evaluate it in our in vitro co-culture model, which supports a chemo-resistant ALL population. We used phospho-protein profiling to evaluate the use of lipid metabolic active compounds in these chemo-resistant cells, due to the up-regulation of multiple active survival signals. In a co-culture with stromal cells, a shift towards anabolic processes occurred, which was further confirmed by assays showing increased lipid content. The treatment of REH leukemia cells with pitavastatin in the co-culture model resulted in significantly higher leukemic cell death than exposure to the standard-of-care chemotherapeutic agent, cytarabine (Ara-C). Our data demonstrates the use of pitavastatin as a possible alternative treatment strategy to improve patient outcomes in chemo-resistant, relapsed ALL.
Keywords: ALL; drug resistance; lipid metabolism; metabolism.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
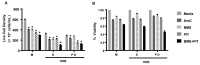
Similar articles
-
Chemotherapeutic Activity of Pitavastatin in Vincristine Resistant B-Cell Acute Lymphoblastic Leukemia.Cancers (Basel). 2023 Jan 24;15(3):707. doi: 10.3390/cancers15030707. Cancers (Basel). 2023. PMID: 36765664 Free PMC article.
-
The MitoNEET Ligand NL-1 Mediates Antileukemic Activity in Drug-Resistant B-Cell Acute Lymphoblastic Leukemia.J Pharmacol Exp Ther. 2019 Jul;370(1):25-34. doi: 10.1124/jpet.118.255984. Epub 2019 Apr 22. J Pharmacol Exp Ther. 2019. PMID: 31010844 Free PMC article.
-
Combination of cabazitaxel and plicamycin induces cell death in drug resistant B-cell acute lymphoblastic leukemia.Leuk Res. 2018 Sep;72:59-66. doi: 10.1016/j.leukres.2018.08.002. Epub 2018 Aug 6. Leuk Res. 2018. PMID: 30103201 Free PMC article.
-
The Bone Marrow Niche in B-Cell Acute Lymphoblastic Leukemia: The Role of Microenvironment from Pre-Leukemia to Overt Leukemia.Int J Mol Sci. 2021 Apr 23;22(9):4426. doi: 10.3390/ijms22094426. Int J Mol Sci. 2021. PMID: 33922612 Free PMC article. Review.
-
Survival of B lineage leukemic cells: signals from the bone marrow microenvironment.Leuk Lymphoma. 2002 Jan;43(1):19-27. doi: 10.1080/10428190210188. Leuk Lymphoma. 2002. PMID: 11908727 Review.
Cited by
-
N-Ethyl-N-Nitrosourea Induced Leukaemia in a Mouse Model: Protective Effect of Icaritin via Inhibition of IL-6/JAK2/STAT3 Pathway Causes Apoptosis.J Inflamm Res. 2024 Feb 7;17:777-790. doi: 10.2147/JIR.S441755. eCollection 2024. J Inflamm Res. 2024. PMID: 38344310 Free PMC article.
-
Molecular Mechanisms Underlying the Anticancer Properties of Pitavastatin against Cervical Cancer Cells.Int J Mol Sci. 2024 Jul 19;25(14):7915. doi: 10.3390/ijms25147915. Int J Mol Sci. 2024. PMID: 39063157 Free PMC article.
References
-
- Mussolin L., Pillon M., Conter V., Piglione M., Lo Nigro L., Pierani P., Micalizzi C., Buffardi S., Basso G., Zanesco L., et al. Prognostic role of minimal residual disease in mature B-cell acute lymphoblastic leukemia of childhood. J. Clin. Oncol. 2007;25:5254–5261. doi: 10.1200/JCO.2007.11.3159. - DOI - PubMed
-
- Conter V., Bartram C.R., Valsecchi M.G., Schrauder A., Panzer-Grumayer R., Moricke A., Arico M., Zimmermann M., Mann G., De Rossi G., et al. Molecular response to treatment redefines all prognostic factors in children and adolescents with B-cell precursor acute lymphoblastic leukemia: Results in 3184 patients of the AIEOP-BFM ALL 2000 study. Blood. 2010;115:3206–3214. doi: 10.1182/blood-2009-10-248146. - DOI - PubMed
-
- Rosales-Rodriguez B., Fernandez-Ramirez F., Nunez-Enriquez J.C., Velazquez-Wong A.C., Medina-Sanson A., Jimenez-Hernandez E., Flores-Lujano J., Penaloza-Gonzalez J.G., Espinosa-Elizondo R.M., Perez-Saldivar M.L., et al. Copy Number Alterations Associated with Acute Lymphoblastic Leukemia in Mexican Children. A report from The Mexican Inter-Institutional Group for the identification of the causes of childhood leukemia. Arch. Med. Res. 2016;47:706–711. doi: 10.1016/j.arcmed.2016.12.002. - DOI - PubMed
-
- Liu J., Masurekar A., Johnson S., Chakraborty S., Griffiths J., Smith D., Alexander S., Dempsey C., Parker C., Harrison S., et al. Stromal cell-mediated mitochondrial redox adaptation regulates drug resistance in childhood acute lymphoblastic leukemia. Oncotarget. 2015;6:43048–43064. doi: 10.18632/oncotarget.5528. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

