Targeting the Subventricular Zone to Promote Myelin Repair in the Aging Brain
- PMID: 35681504
- PMCID: PMC9180001
- DOI: 10.3390/cells11111809
Targeting the Subventricular Zone to Promote Myelin Repair in the Aging Brain
Abstract
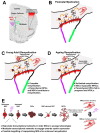
The subventricular zone (SVZ) is the largest and most active germinal zone in the adult forebrain. Neural stem cells (NSCs) of the SVZ generate olfactory interneurons throughout life and retain the intrinsic ability to generate oligodendrocytes (OLs), the myelinating cells of the central nervous system. OLs and myelin are targets in demyelinating diseases such as multiple sclerosis (MS). Remyelination is dependent on the ability of oligodendrocyte progenitor cells (OPCs) to proliferate, migrate, and terminally differentiate into myelinating OLs. During aging, there is a gradual decrease in the regenerative capacity of OPCs, and the consequent loss of OLs and myelin is a contributing factor in cognitive decline and the failure of remyelination in MS and other pathologies with aging contexts, including Alzheimer's disease (AD) and stroke. The age-related decrease in oligodendrogenesis has not been fully characterised but is known to reflect changes in intrinsic and environmental factors affecting the ability of OPCs to respond to pro-differentiation stimuli. Notably, SVZ-derived OPCs are an important source of remyelinating OLs in addition to parenchymal OPCs. In this mini-review, we briefly discuss differences between SVZ-derived and parenchymal OPCs in their responses to demyelination and highlight challenges associated with their study in vivo and how they can be targeted for regenerative therapies in the aged brain.
Keywords: aging; multiple sclerosis; oligodendrogenesis; remyelination; subventricular zone.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
Similar articles
-
Neural Stem Cells of the Subventricular Zone Contribute to Neuroprotection of the Corpus Callosum after Cuprizone-Induced Demyelination.J Neurosci. 2019 Jul 10;39(28):5481-5492. doi: 10.1523/JNEUROSCI.0227-18.2019. Epub 2019 May 28. J Neurosci. 2019. PMID: 31138656 Free PMC article.
-
Sequential Contribution of Parenchymal and Neural Stem Cell-Derived Oligodendrocyte Precursor Cells toward Remyelination.Neuroglia. 2018 Sep;1(1):91-105. doi: 10.3390/neuroglia1010008. Epub 2018 Jun 12. Neuroglia. 2018. PMID: 30662979 Free PMC article.
-
Role of Oligodendrocyte Dysfunction in Demyelination, Remyelination and Neurodegeneration in Multiple Sclerosis.Adv Exp Med Biol. 2017;958:91-127. doi: 10.1007/978-3-319-47861-6_7. Adv Exp Med Biol. 2017. PMID: 28093710 Review.
-
Oligodendrogenesis from neural stem cells: perspectives for remyelinating strategies.Int J Dev Neurosci. 2013 Nov;31(7):692-700. doi: 10.1016/j.ijdevneu.2013.01.004. Epub 2013 Jan 20. Int J Dev Neurosci. 2013. PMID: 23340483 Review.
-
Lack of astrocytes hinders parenchymal oligodendrocyte precursor cells from reaching a myelinating state in osmolyte-induced demyelination.Acta Neuropathol Commun. 2020 Dec 24;8(1):224. doi: 10.1186/s40478-020-01105-2. Acta Neuropathol Commun. 2020. PMID: 33357244 Free PMC article.
Cited by
-
Olive- and Coconut-Oil-Enriched Diets Decreased Secondary Bile Acids and Regulated Metabolic and Transcriptomic Markers of Brain Injury in the Frontal Cortexes of NAFLD Pigs.Brain Sci. 2022 Sep 4;12(9):1193. doi: 10.3390/brainsci12091193. Brain Sci. 2022. PMID: 36138929 Free PMC article.
-
Frontiers in Neurogenesis.Cells. 2022 Nov 11;11(22):3567. doi: 10.3390/cells11223567. Cells. 2022. PMID: 36428996 Free PMC article.
-
Neural stem cells and oligodendrocyte progenitor cells compete for remyelination in the corpus callosum.Front Cell Neurosci. 2023 Jan 26;17:1114781. doi: 10.3389/fncel.2023.1114781. eCollection 2023. Front Cell Neurosci. 2023. PMID: 36779010 Free PMC article.
-
In-vivo characterization of macro- and microstructural injury of the subventricular zone in relapsing-remitting and progressive multiple sclerosis.Front Neurosci. 2023 Apr 11;17:1112199. doi: 10.3389/fnins.2023.1112199. eCollection 2023. Front Neurosci. 2023. PMID: 37113155 Free PMC article.
-
The Genomic Intersection of Oligodendrocyte Dynamics in Schizophrenia and Aging Unravels Novel Pathological Mechanisms and Therapeutic Potentials.Int J Mol Sci. 2024 Apr 18;25(8):4452. doi: 10.3390/ijms25084452. Int J Mol Sci. 2024. PMID: 38674040 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

