The Extracellular Matrix Proteins Tenascin-C and Tenascin-R Retard Oligodendrocyte Precursor Maturation and Myelin Regeneration in a Cuprizone-Induced Long-Term Demyelination Animal Model
- PMID: 35681468
- PMCID: PMC9179356
- DOI: 10.3390/cells11111773
The Extracellular Matrix Proteins Tenascin-C and Tenascin-R Retard Oligodendrocyte Precursor Maturation and Myelin Regeneration in a Cuprizone-Induced Long-Term Demyelination Animal Model
Abstract
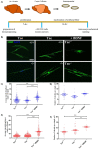
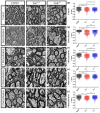
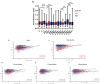
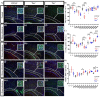
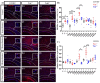
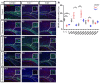
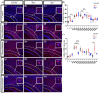
Oligodendrocytes are the myelinating cells of the central nervous system. The physiological importance of oligodendrocytes is highlighted by diseases such as multiple sclerosis, in which the myelin sheaths are degraded and the axonal signal transmission is compromised. In a healthy brain, spontaneous remyelination is rare, and newly formed myelin sheaths are thinner and shorter than the former ones. The myelination process requires the migration, proliferation, and differentiation of oligodendrocyte precursor cells (OPCs) and is influenced by proteins of the extracellular matrix (ECM), which consists of a network of glycoproteins and proteoglycans. In particular, the glycoprotein tenascin-C (Tnc) has an inhibitory effect on the differentiation of OPCs and the remyelination efficiency of oligodendrocytes. The structurally similar tenascin-R (Tnr) exerts an inhibitory influence on the formation of myelin membranes in vitro. When Tnc knockout oligodendrocytes were applied to an in vitro myelination assay using artificial fibers, a higher number of sheaths per single cell were obtained compared to the wild-type control. This effect was enhanced by adding brain-derived neurotrophic factor (BDNF) to the culture system. Tnr-/- oligodendrocytes behaved differently in that the number of formed sheaths per single cell was decreased, indicating that Tnr supports the differentiation of OPCs. In order to study the functions of tenascin proteins in vivo Tnc-/- and Tnr-/- mice were exposed to Cuprizone-induced demyelination for a period of 10 weeks. Both Tnc-/- and Tnr-/- mouse knockout lines displayed a significant increase in the regenerating myelin sheath thickness after Cuprizone treatment. Furthermore, in the absence of either tenascin, the number of OPCs was increased. These results suggest that the fine-tuning of myelin regeneration is regulated by the major tenascin proteins of the CNS.
Keywords: cuprizone; myelin; myelin lesion; oligodendrocyte; tenascin-C; tenascin-R.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be constructed as a potential conflict of interest.
Figures

Similar articles
-
Tenascins Interfere With Remyelination in an Ex Vivo Cerebellar Explant Model of Demyelination.Front Cell Dev Biol. 2022 Mar 15;10:819967. doi: 10.3389/fcell.2022.819967. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35372366 Free PMC article.
-
Tenascin C and tenascin R similarly prevent the formation of myelin membranes in a RhoA-dependent manner, but antagonistically regulate the expression of myelin basic protein via a separate pathway.Glia. 2009 Dec;57(16):1790-801. doi: 10.1002/glia.20891. Glia. 2009. PMID: 19459213
-
Inactivation of Protein Tyrosine Phosphatase Receptor Type Z by Pleiotrophin Promotes Remyelination through Activation of Differentiation of Oligodendrocyte Precursor Cells.J Neurosci. 2015 Sep 2;35(35):12162-71. doi: 10.1523/JNEUROSCI.2127-15.2015. J Neurosci. 2015. PMID: 26338327 Free PMC article.
-
Engineering biomaterial microenvironments to promote myelination in the central nervous system.Brain Res Bull. 2019 Oct;152:159-174. doi: 10.1016/j.brainresbull.2019.07.013. Epub 2019 Jul 12. Brain Res Bull. 2019. PMID: 31306690 Review.
-
Matrix metalloproteinases shape the oligodendrocyte (niche) during development and upon demyelination.Neurosci Lett. 2020 Jun 11;729:134980. doi: 10.1016/j.neulet.2020.134980. Epub 2020 Apr 19. Neurosci Lett. 2020. PMID: 32315713 Review.
Cited by
-
Insights into the Pathobiology of GM1 Gangliosidosis from Single-Nucleus Transcriptomic Analysis of CNS Cells in a Mouse Model.Int J Mol Sci. 2024 Sep 8;25(17):9712. doi: 10.3390/ijms25179712. Int J Mol Sci. 2024. PMID: 39273659 Free PMC article.
-
Substrate-bound and soluble domains of tenascin-C regulate differentiation, proliferation and migration of neural stem and progenitor cells.Front Cell Neurosci. 2024 Feb 14;18:1357499. doi: 10.3389/fncel.2024.1357499. eCollection 2024. Front Cell Neurosci. 2024. PMID: 38425428 Free PMC article.
-
CAQK, a peptide associating with extracellular matrix components targets sites of demyelinating injuries.Front Cell Neurosci. 2022 Aug 22;16:908401. doi: 10.3389/fncel.2022.908401. eCollection 2022. Front Cell Neurosci. 2022. PMID: 36072569 Free PMC article.
-
Biology of Tenascin C and its Role in Physiology and Pathology.Curr Med Chem. 2024;31(19):2706-2731. doi: 10.2174/0929867330666230404124229. Curr Med Chem. 2024. PMID: 37021423 Review.
-
Myelin sheath injury and repairment after subarachnoid hemorrhage.Front Pharmacol. 2023 Apr 3;14:1145605. doi: 10.3389/fphar.2023.1145605. eCollection 2023. Front Pharmacol. 2023. PMID: 37077816 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

