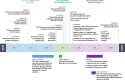
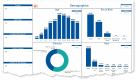
Standardizing, harmonizing, and protecting data collection to broaden the impact of COVID-19 research: the rapid acceleration of diagnostics-underserved populations (RADx-UP) initiative
- PMID: 35678579
- PMCID: PMC9382379
- DOI: 10.1093/jamia/ocac097
Standardizing, harmonizing, and protecting data collection to broaden the impact of COVID-19 research: the rapid acceleration of diagnostics-underserved populations (RADx-UP) initiative
Abstract
Objective: The Rapid Acceleration of Diagnostics-Underserved Populations (RADx-UP) program is a consortium of community-engaged research projects with the goal of increasing access to Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) tests in underserved populations. To accelerate clinical research, common data elements (CDEs) were selected and refined to standardize data collection and enhance cross-consortium analysis.
Materials and methods: The RADx-UP consortium began with more than 700 CDEs from the National Institutes of Health (NIH) CDE Repository, Disaster Research Response (DR2) guidelines, and the PHENotypes and eXposures (PhenX) Toolkit. Following a review of initial CDEs, we made selections and further refinements through an iterative process that included live forums, consultations, and surveys completed by the first 69 RADx-UP projects.
Results: Following a multistep CDE development process, we decreased the number of CDEs, modified the question types, and changed the CDE wording. Most research projects were willing to collect and share demographic NIH Tier 1 CDEs, with the top exception reason being a lack of CDE applicability to the project. The NIH RADx-UP Tier 1 CDE with the lowest frequency of collection and sharing was sexual orientation.
Discussion: We engaged a wide range of projects and solicited bidirectional input to create CDEs. These RADx-UP CDEs could serve as the foundation for a patient-centered informatics architecture allowing the integration of disease-specific databases to support hypothesis-driven clinical research in underserved populations.
Conclusion: A community-engaged approach using bidirectional feedback can lead to the better development and implementation of CDEs in underserved populations during public health emergencies.
Keywords: common data elements; community research; data privacy; health equity; underserved populations.
© The Author(s) 2022. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Figures
Similar articles
-
RADx-UP Testing Core: Access to COVID-19 Diagnostics in Community-Engaged Research with Underserved Populations.J Clin Microbiol. 2023 Aug 23;61(8):e0036723. doi: 10.1128/jcm.00367-23. Epub 2023 Jul 3. J Clin Microbiol. 2023. PMID: 37395655 Free PMC article.
-
Qualitative Evaluation of RADx-UP Projects Addressing COVID-19 Testing Disparities Among Underserved Populations.Am J Public Health. 2024 May;114(S5):S410-S415. doi: 10.2105/AJPH.2024.307632. Epub 2024 Mar 28. Am J Public Health. 2024. PMID: 38547469 Free PMC article.
-
National Institute of Neurological Disorders and Stroke Common Data Element Project - approach and methods.Clin Trials. 2012 Jun;9(3):322-9. doi: 10.1177/1740774512438980. Epub 2012 Feb 27. Clin Trials. 2012. PMID: 22371630 Free PMC article.
-
Common Data Elements for Unruptured Intracranial Aneurysms and Subarachnoid Hemorrhage Clinical Research: A National Institute for Neurological Disorders and Stroke and National Library of Medicine Project.Neurocrit Care. 2019 Jun;30(Suppl 1):4-19. doi: 10.1007/s12028-019-00723-6. Neurocrit Care. 2019. PMID: 31087257
-
A common data language for clinical research studies: the National Institute of Neurological Disorders and Stroke and American Academy for Cerebral Palsy and Developmental Medicine Cerebral Palsy Common Data Elements Version 1.0 recommendations.Dev Med Child Neurol. 2018 Oct;60(10):976-986. doi: 10.1111/dmcn.13723. Epub 2018 Mar 15. Dev Med Child Neurol. 2018. PMID: 29542813 Review.
Cited by
-
Factors Associated with COVID-19 Vaccination Uptake in Great Plains American Indian Communities.J Racial Ethn Health Disparities. 2024 Dec;11(6):3690-3703. doi: 10.1007/s40615-023-01818-9. Epub 2023 Oct 5. J Racial Ethn Health Disparities. 2024. PMID: 37796431
-
Continuing the journey toward semantic interoperability in clinical care and biomedical and health research.J Am Med Inform Assoc. 2022 Aug 16;29(9):1447-1448. doi: 10.1093/jamia/ocac128. J Am Med Inform Assoc. 2022. PMID: 35975586 Free PMC article. No abstract available.
-
REPRESENT recommendations: improving inclusion and trust in cancer early detection research.Br J Cancer. 2023 Oct;129(8):1195-1208. doi: 10.1038/s41416-023-02414-8. Epub 2023 Sep 9. Br J Cancer. 2023. PMID: 37689805 Free PMC article. Review.
-
Drug use and COVID-19 testing, vaccination, and infection among underserved, minority communities in Miami, Florida.PLoS One. 2024 Apr 30;19(4):e0297327. doi: 10.1371/journal.pone.0297327. eCollection 2024. PLoS One. 2024. PMID: 38687734 Free PMC article.
-
Examining COVID-19 testing and vaccination behaviors by heritage and linguistic preferences among Hispanic, Latino, or Spanish RADx-UP participants.Prev Med Rep. 2023 Aug 2;35:102359. doi: 10.1016/j.pmedr.2023.102359. eCollection 2023 Oct. Prev Med Rep. 2023. PMID: 37584063 Free PMC article.
References
Publication types
MeSH terms
Grants and funding
- U24 MD016258/MD/NIMHD NIH HHS/United States
- U24 MD016258/GF/NIH HHS/United States
- 1U24-MD016258/MD/NIMHD NIH HHS/United States
- CC2-Duke-2016/PCORI/Patient-Centered Outcomes Research Institute/United States
- NU38OT000316/CC/CDC HHS/United States
- HHSN272201500006I/National Institute of Allergy and Infectious Diseases
- HHSN275201000003I/HD/NICHD NIH HHS/United States
- 1U24-AT010961/GF/NIH HHS/United States
- 1UC2-CA233254/CA/NCI NIH HHS/United States
- 6292-2020-DQ/5U01-FD006292/Medical Device Innovation Consortium
- 75F40119C10086/FD/FDA HHS/United States
- 5U18-FD006298/U.S. Food and Drug Administration
- 1RM1-HG011123/HG/NHGRI NIH HHS/United States
- 75FCMC18D0047/Centers for Medicare & Medicaid Service
- 5UL1-TR002553/TR/NCATS NIH HHS/United States
- 1U24MD016258/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous