Viral G Protein-Coupled Receptors Encoded by β- and γ-Herpesviruses
- PMID: 35671566
- PMCID: PMC9584139
- DOI: 10.1146/annurev-virology-100220-113942
Viral G Protein-Coupled Receptors Encoded by β- and γ-Herpesviruses
Abstract
Herpesviruses are ancient large DNA viruses that have exploited gene capture as part of their strategy to escape immune surveillance, promote virus spreading, or reprogram host cells to benefit their survival. Most acquired genes are transmembrane proteins and cytokines, such as viral G protein-coupled receptors (vGPCRs), chemokines, and chemokine-binding proteins. This review focuses on the vGPCRs encoded by the human β- and γ-herpesviruses. These include receptors from human cytomegalovirus, which encodes four vGPCRs: US27, US28, UL33, and UL78; human herpesvirus 6 and 7 with two receptors: U12 and U51; Epstein-Barr virus with one: BILF1; and Kaposi's sarcoma-associated herpesvirus with one: open reading frame 74, ORF74. We discuss ligand binding, signaling, and structures of the vGPCRs in light of robust differences from endogenous receptors. Finally, we briefly discuss the therapeutic targeting of vGPCRs as future treatment of acute and chronic herpesvirus infections.
Keywords: G protein signaling; GPCR structure; broad-spectrum ligand binding; chemokine receptor; herpesvirus.
Figures
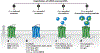

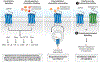

Similar articles
-
Herpesvirus-encoded GPCRs rewire cellular signaling.Mol Cell Endocrinol. 2011 Jan 15;331(2):179-84. doi: 10.1016/j.mce.2010.04.007. Epub 2010 Apr 14. Mol Cell Endocrinol. 2011. PMID: 20398729 Review.
-
Pharmacological and biochemical characterization of human cytomegalovirus-encoded G protein-coupled receptors.Methods Enzymol. 2009;460:151-71. doi: 10.1016/S0076-6879(09)05207-0. Methods Enzymol. 2009. PMID: 19446724
-
HCMV-encoded G-protein-coupled receptors as constitutively active modulators of cellular signaling networks.Trends Pharmacol Sci. 2006 Jan;27(1):56-63. doi: 10.1016/j.tips.2005.11.006. Epub 2005 Dec 13. Trends Pharmacol Sci. 2006. PMID: 16352349 Review.
-
Viral chemokine receptors and chemokines in human cytomegalovirus trafficking and interaction with the immune system. CMV chemokine receptors.Curr Top Microbiol Immunol. 2002;269:203-34. doi: 10.1007/978-3-642-59421-2_13. Curr Top Microbiol Immunol. 2002. PMID: 12224510 Review.
-
Constitutive activation of T cells by γ2-herpesviral GPCR through the interaction with cellular CXCR4.Biochim Biophys Acta Mol Cell Res. 2017 Jan;1864(1):1-11. doi: 10.1016/j.bbamcr.2016.10.008. Epub 2016 Oct 15. Biochim Biophys Acta Mol Cell Res. 2017. PMID: 27751885
Cited by
-
Structural insights into KSHV-GPCR constitutive activation and CXCL1 chemokine recognition.Proc Natl Acad Sci U S A. 2024 Oct 15;121(42):e2403217121. doi: 10.1073/pnas.2403217121. Epub 2024 Oct 8. Proc Natl Acad Sci U S A. 2024. PMID: 39378089
-
A homotrimeric GPCR architecture of the human cytomegalovirus revealed by cryo-EM.Cell Discov. 2024 May 16;10(1):52. doi: 10.1038/s41421-024-00684-x. Cell Discov. 2024. PMID: 38750025 Free PMC article. No abstract available.
-
The chemokine receptor CCR5: multi-faceted hook for HIV-1.Retrovirology. 2024 Jan 23;21(1):2. doi: 10.1186/s12977-024-00634-1. Retrovirology. 2024. PMID: 38263120 Free PMC article. Review.
-
The US21 viroporin of human cytomegalovirus stimulates cell migration and adhesion.mBio. 2023 Aug 31;14(4):e0074923. doi: 10.1128/mbio.00749-23. Epub 2023 Jul 21. mBio. 2023. PMID: 37477430 Free PMC article.
-
New genetic and epigenetic insights into the chemokine system: the latest discoveries aiding progression toward precision medicine.Cell Mol Immunol. 2023 Jul;20(7):739-776. doi: 10.1038/s41423-023-01032-x. Epub 2023 May 17. Cell Mol Immunol. 2023. PMID: 37198402 Free PMC article. Review.
References
-
- Rosenkilde MM, Benned-Jensen T, Andersen H, Holst PJ, Kledal TN, et al. 2006. Molecular pharmacological phenotyping of EBI2. An orphan seven-transmembrane receptor with constitutive activity. J. Biol. Chem 281:13199–208 - PubMed
-
- Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, et al. 1997. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J. Biol. Chem 272:13803–9 - PubMed
-
- Liu C, Yang XV, Wu J, Kuei C, Mani NS, et al. 2011. Oxysterols direct B-cell migration through EBI2. Nature 475:519–23 - PubMed
-
- Rosenkilde MM, Kledal TN. 2006. Targeting herpesvirus reliance of the chemokine system. Curr. Drug Targets 7:103–18 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

