PPAR- γ Agonist Pioglitazone Restored Mouse Liver mRNA Expression of Clock Genes and Inflammation-Related Genes Disrupted by Reversed Feeding
- PMID: 35663475
- PMCID: PMC9162826
- DOI: 10.1155/2022/7537210
PPAR- γ Agonist Pioglitazone Restored Mouse Liver mRNA Expression of Clock Genes and Inflammation-Related Genes Disrupted by Reversed Feeding
Abstract
Introduction: The master clock, which is located in the suprachiasmatic nucleus (SCN), harmonizes clock genes present in the liver to synchronize life rhythms and bioactivity with the surrounding environment. The reversed feeding disrupts the expression of clock genes in the liver. Recently, a novel role of PPAR-γ as a regulator in correlating circadian rhythm and metabolism was demonstrated. This study examined the influence of PPAR-γ agonist pioglitazone (PG) on the mRNA expression profile of principle clock genes and inflammation-related genes in the mouse liver disrupted by reverse feeding.
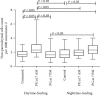
Methods: Mice were randomly assigned to daytime-feeding and nighttime-feeding groups. Mice in daytime-feeding groups received food from 7 AM to 7 PM, and mice in nighttime-feeding groups received food from 7 PM to 7 AM. PG was administered in the dose of 20 mg/kg per os as aqueous suspension 40 μl at 7 AM or 7 PM. Each group consisted of 12 animals. On day 8 of the feeding intervention, mice were sacrificed by cervical dislocation at noon (05 hours after light onset (HALO)) and midnight (HALO 17). Liver expressions of Bmal1, Clock, Rev-erb alpha, Cry1, Cry2, Per1, Per2, Cxcl5, Nrf2, and Ppar-γ were determined by quantitative reverse transcription PCR. Liver expression of PPAR-γ, pNF-κB, and IL-6 was determined by Western blotting. Glucose, ceruloplasmin, total cholesterol, triglyceride concentrations, and ALT and AST activities were measured in sera by photometric methods. The null hypothesis tested was that PG and the time of its administration have no influence on the clock gene expression impaired by reverse feeding.
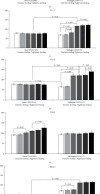
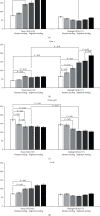
Results: Administration of PG at 7 AM to nighttime-feeding mice did not reveal any influence on the expression of the clock or inflammation-related genes either at midnight or at noon. In the daytime-feeding group, PG intake at 7 PM led to an increase in Per2 and Rev-erb alpha mRNA at noon, an increase in Ppar-γ mRNA at midnight, and a decrease in Nfκb (p65) mRNA at noon. In general, PG administration at 7 PM slightly normalized the impaired expression of clock genes and increased anti-inflammatory potency impaired by reversed feeding. This pattern was supported by biochemical substrate levels-glucose, total cholesterol, ALT, and AST activities. The decrease in NF-κB led to the inhibition of serum ceruloplasmin levels as well as IL-6 in liver tissue. According to our data, PG intake at 7 PM exerts strong normalization of clock gene expression with a further increase in Nrf2 and, especially, Ppar-γ and PPAR-γ expression with inhibition of Nfκb and pNF-κB expression in daytime-feeding mice. These expression changes resulted in decreased hyperglycemia, hypercholesterolemia, ALT, and AST activities. Thus, PG had a potent chronopharmacological effect when administered at 7 PM to daytime-feeding mice.
Conclusions: Our study indicates that reversed feeding induced the disruption of mouse liver circadian expression pattern of clock genes accompanied by increasing Nfκb and pNF-κB and IL-6 expression and decreasing Nrf2 and PPAR-γ. Administration of PG restored the clock gene expression profile and decreased Nfκb, pNF-κB, and IL-6, as well as increased Nrf2, Ppar-γ, and PPAR-γ expression. PG intake at 7 PM was more effective than at 7 AM in reversed feeding mice.
Copyright © 2022 T. Fedchenko et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures


Similar articles
-
PPARG agonist pioglitazone influences diurnal kidney medulla mRNA expression of core clock, inflammation-, and metabolism-related genes disrupted by reverse feeding in mice.Physiol Rep. 2022 Dec;10(23):e15535. doi: 10.14814/phy2.15535. Physiol Rep. 2022. PMID: 36511486 Free PMC article.
-
Uncoupling of peripheral and master clock gene rhythms by reversed feeding leads to an exacerbated inflammatory response after polymicrobial sepsis in mice.Shock. 2014 Mar;41(3):214-21. doi: 10.1097/SHK.0000000000000094. Shock. 2014. PMID: 24300828
-
PPARG stimulation restored lung mRNA expression of core clock, inflammation- and metabolism-related genes disrupted by reversed feeding in male mice.Physiol Rep. 2023 Sep;11(17):e15823. doi: 10.14814/phy2.15823. Physiol Rep. 2023. PMID: 37704580 Free PMC article.
-
Melatonin feedback on clock genes: a theory involving the proteasome.J Pineal Res. 2015 Jan;58(1):1-11. doi: 10.1111/jpi.12189. Epub 2014 Nov 22. J Pineal Res. 2015. PMID: 25369242 Review.
-
Circadian Rhythms in Diet-Induced Obesity.Adv Exp Med Biol. 2017;960:19-52. doi: 10.1007/978-3-319-48382-5_2. Adv Exp Med Biol. 2017. PMID: 28585194 Review.
Cited by
-
Ameliorative effect of Nigella sativa conjugated silver nanoparticles against chromium-induced hepatotoxicity and renal toxicity in mice.Saudi J Biol Sci. 2023 Mar;30(3):103571. doi: 10.1016/j.sjbs.2023.103571. Epub 2023 Jan 25. Saudi J Biol Sci. 2023. PMID: 36844642 Free PMC article.
-
Timing Is Important-Management of Metabolic Syndrome According to the Circadian Rhythm.Biomedicines. 2023 Apr 13;11(4):1171. doi: 10.3390/biomedicines11041171. Biomedicines. 2023. PMID: 37189789 Free PMC article. Review.
-
Circadian Dysfunction in Adipose Tissue: Chronotherapy in Metabolic Diseases.Biology (Basel). 2023 Aug 2;12(8):1077. doi: 10.3390/biology12081077. Biology (Basel). 2023. PMID: 37626963 Free PMC article. Review.
-
Expression of periferal core molecular clock genes in oral mucosa depends on the chronotype in patients with maxillofacial cellulitis.J Oral Biol Craniofac Res. 2023 Sep-Oct;13(5):517-521. doi: 10.1016/j.jobcr.2023.06.001. Epub 2023 Jun 16. J Oral Biol Craniofac Res. 2023. PMID: 37361670 Free PMC article.
-
PPARG agonist pioglitazone influences diurnal kidney medulla mRNA expression of core clock, inflammation-, and metabolism-related genes disrupted by reverse feeding in mice.Physiol Rep. 2022 Dec;10(23):e15535. doi: 10.14814/phy2.15535. Physiol Rep. 2022. PMID: 36511486 Free PMC article.
References
-
- Damiola F., Le Minh N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes & Development . 2000;14(23):2950–2961. doi: 10.1101/gad.183500. - DOI - PMC - PubMed
-
- Mukherji A., Kobiita A., Chambon P. Shifting the feeding of mice to the rest phase creates metabolic alterations, which, on their own, shift the peripheral circadian clocks by 12 hours. Proceedings of the National Academy of Sciences of the United States of America . 2015;112(48):E6683–E6690. doi: 10.1073/pnas.1519735112. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

