Retrospective Analysis of Real-World Management of EGFR-Mutated Advanced NSCLC, After First-Line EGFR-TKI Treatment: US Treatment Patterns, Attrition, and Survival Data
- PMID: 35661118
- PMCID: PMC9392819
- DOI: 10.1007/s40801-022-00302-w
Retrospective Analysis of Real-World Management of EGFR-Mutated Advanced NSCLC, After First-Line EGFR-TKI Treatment: US Treatment Patterns, Attrition, and Survival Data
Abstract
Background and objectives: Epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) are standard-of-care first-line (1L) treatment for EGFR mutation-positive advanced/metastatic non-small cell lung cancer. In 2015, osimertinib, a third-generation EGFR-TKI, received US accelerated approval for second-line (2L) EGFR T790M-positive non-small cell lung cancer treatment. The objective of this US study was to characterize treatment patterns, attrition, and survival in EGFR mutation-positive non-small cell lung cancer, after 1L first-/second-generation EGFR-TKI treatment.
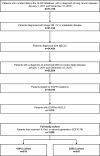
Methods: We retrospectively analyzed 1029 patients diagnosed with stage IIIB/IV non-small cell lung cancer from 1 January, 2011 to 31 December, 2018 using the US electronic medical record CancerLinQ Discovery® database. Demographic/disease characteristics, EGFR mutations, treatments, and death dates were collected.
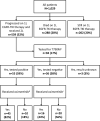
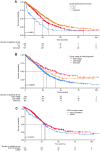
Results: From 1 January, 2011 to 31 December, 2014 (< 2015 cohort), 519 patients received 1L EGFR-TKIs and 510 between 1 January, 2015 and 31 December, 2018 (≥ 2015 cohort). Median follow-up from advanced diagnosis was 19.8 months (interquartile range: 9.9-33.4 months). Twenty-eight percent of patients (288/1029) died without receiving 2L, and 52% (539/1029) initiated 2L with 35% (186/539) receiving osimertinib; in the < 2015 and ≥ 2015 cohorts, the same proportion initiated 2L (52%; 272/519 vs 267/510, respectively). Median overall survival from advanced diagnosis for patients initially diagnosed with stage I-IIIA disease was 43.3 months (95% confidence interval 30.9-73.7), vs 26.4 months (95% confidence interval 24.4-28.1) for stage IIIB-IV; all-cause mortality hazard ratio: 1.56 (95% confidence interval 1.2-2.0; p = 0.001).
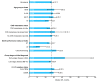
Conclusions: We identified disease stage, performance status, and central nervous system metastasis as survival predictors, highlighting the importance of optimal 1L treatment selection. Over a quarter of patients died before initiating 2L; half progressed after 1L and received 2L, of whom a third received 2L osimertinib.
© 2022. The Author(s).
Conflict of interest statement
J. Nieva reports ownership of stock/shares for Cansera, Epic Sciences, and Quantgene and has received honoraria from AstraZeneca, Fujirebio, Genentech, Western Oncolytics, Takeda, and Amgen. J. Nieva has received research grants/funds from Genentech and Merck & Co. K. Reckamp received personal fees for consulting from Amgen, AstraZeneca, Blueprint, Boehringer Ingelheim, Calithera, Euclises, Genentech, Guardant, Janssen, Lilly, Merck KGA, Precision Health, Seattle Genetics, Takeda, and Tesaro. K. Reckamp received (institution) research grants/funds from AbbVie, Acea, Adaptimmune, Boehringer Ingelheim, Bristol Myers Squibb, Genentech, GlaxoSmithKline, Guardant, Janssen, Loxo Oncology, Molecular Partners, Seattle Genetics, Spectrum, Takeda, Xcovery, and Zeno. D. Potter was an employee of AstraZeneca when this research was conducted. D. Potter joined ASCO and CancerLinQ after participating in this study. This study and related conclusions reflect the independent work of study authors and do not necessarily represent the views of ASCO or CancerLinQ. A. Taylor and P. Sun are employees of AstraZeneca and report ownership of stocks/shares. Medical writing support for the development of this article, under the direction of the authors, was provided by Preeyah Purang, BSc and Gemma White, MSc, of Ashfield MedComms UK, an Ashfield Health company, and was funded by AstraZeneca.
Figures

Similar articles
-
Observational Study of Treatment Patterns in Patients with Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Non-Small Cell Lung Cancer After First-Line EGFR-Tyrosine Kinase Inhibitors.Adv Ther. 2020 Feb;37(2):946-954. doi: 10.1007/s12325-020-01221-4. Epub 2020 Jan 18. Adv Ther. 2020. PMID: 31955357
-
Real-world treatment patterns of metastatic non-small cell lung cancer patients receiving epidermal growth factor receptor tyrosine kinase inhibitors.Cancer Med. 2023 Jan;12(1):159-169. doi: 10.1002/cam4.4918. Epub 2022 Jun 15. Cancer Med. 2023. PMID: 35702932 Free PMC article.
-
Real-world outcomes, treatment patterns and T790M testing rates in non-small cell lung cancer patients treated with first-line first- or second-generation epidermal growth factor receptor tyrosine kinase inhibitors from the Slovenian cohort of the REFLECT study.Radiol Oncol. 2022 Aug 14;56(3):371-379. doi: 10.2478/raon-2022-0025. Radiol Oncol. 2022. PMID: 35853681 Free PMC article.
-
Overall Treatment Strategy for Patients With Metastatic NSCLC With Activating EGFR Mutations.Clin Lung Cancer. 2022 Jan;23(1):e69-e82. doi: 10.1016/j.cllc.2021.10.009. Epub 2021 Oct 25. Clin Lung Cancer. 2022. PMID: 34865963 Review.
-
Ideal sequencing in Stage IV epidermal growth factor receptor mutant Non-Small-Cell Lung Cancer.Indian J Cancer. 2022 Mar;59(Supplement):S80-S89. doi: 10.4103/ijc.IJC_50_21. Indian J Cancer. 2022. PMID: 35343193 Review.
Cited by
-
Characteristics of Long-Term Survivors With EGFR-Mutant Metastatic NSCLC.JTO Clin Res Rep. 2024 Mar 26;5(8):100669. doi: 10.1016/j.jtocrr.2024.100669. eCollection 2024 Aug. JTO Clin Res Rep. 2024. PMID: 39157674 Free PMC article.
-
Real-World Analysis of Post-Progression Treatment Patterns and Outcomes for EGFR Mutation-Positive Patients Treated with First-Line Osimertinib.Curr Oncol. 2024 Apr 26;31(5):2427-2440. doi: 10.3390/curroncol31050182. Curr Oncol. 2024. PMID: 38785463 Free PMC article.
-
A Randomized, Multi-Center, Open Label Study to Compare the Safety and Efficacy between Afatinib Monotherapy and Combination Therapy with HAD-B1 for the Locally Advanced or Metastatic NSCLC Patients with EGFR Mutations.Integr Cancer Ther. 2024 Jan-Dec;23:15347354241268231. doi: 10.1177/15347354241268231. Integr Cancer Ther. 2024. PMID: 39103991 Free PMC article. Clinical Trial.
-
The Treatment of a New Entity in Advanced Non-small Cell Lung Cancer: MET Exon 14 Skipping Mutation.Curr Med Chem. 2024;31(21):3043-3056. doi: 10.2174/0929867331666230803094432. Curr Med Chem. 2024. PMID: 37534484 Review.
-
Real-World Outcomes of Patients with Advanced Epidermal Growth Factor Receptor-Mutated Non-Small Cell Lung Cancer in Canada Using Data Extracted by Large Language Model-Based Artificial Intelligence.Curr Oncol. 2024 Apr 2;31(4):1947-1960. doi: 10.3390/curroncol31040146. Curr Oncol. 2024. PMID: 38668049 Free PMC article.
References
-
- National Cancer Institute. Lung and bronchus cancer. Long-term trends in SEER age-adjusted incidence rates, 1975–2017. By sex, all races (includes Hispanic), all ages, delay-adjusted rates. SEER*Explorer. https://seer.cancer.gov/explorer/application.html?site=47&data_type=1&gr.... Accessed 27 Nov 2020.
-
- National Cancer Institute. Lung and bronchus cancer. Stage distribution of SEER incidence cases, 2008–2017. By sex, all races (includes Hispanic), all ages. SEER*Explorer. https://seer.cancer.gov/explorer/application.html?site=47&data_type=1&gr.... Accessed 27 Nov 2020.
-
- Planchard D, Popat S, Kerr K, Novello S, Smit EF, Faivre-Finn C, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up (2020 update). https://www.esmo.org/guidelines/lung-and-chest-tumours/clinical-practice.... Accessed Apr 2021. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

