Exercise, Mitohormesis, and Mitochondrial ORF of the 12S rRNA Type-C (MOTS-c)
- PMID: 35656563
- PMCID: PMC9171157
- DOI: 10.4093/dmj.2022.0092
Exercise, Mitohormesis, and Mitochondrial ORF of the 12S rRNA Type-C (MOTS-c)
Abstract
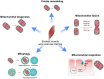
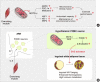
Low levels of mitochondrial stress are beneficial for organismal health and survival through a process known as mitohormesis. Mitohormetic responses occur during or after exercise and may mediate some salutary effects of exercise on metabolism. Exercise-related mitohormesis involves reactive oxygen species production, mitochondrial unfolded protein response (UPRmt), and release of mitochondria-derived peptides (MDPs). MDPs are a group of small peptides encoded by mitochondrial DNA with beneficial metabolic effects. Among MDPs, mitochondrial ORF of the 12S rRNA type-c (MOTS-c) is the most associated with exercise. MOTS-c expression levels increase in skeletal muscles, systemic circulation, and the hypothalamus upon exercise. Systemic MOTS-c administration increases exercise performance by boosting skeletal muscle stress responses and by enhancing metabolic adaptation to exercise. Exogenous MOTS-c also stimulates thermogenesis in subcutaneous white adipose tissues, thereby enhancing energy expenditure and contributing to the anti-obesity effects of exercise training. This review briefly summarizes the mitohormetic mechanisms of exercise with an emphasis on MOTS-c.
Keywords: Exercise; Hormesis; MOTS-c peptide, human; Mitochondria; Mitochondrial proteins; Obesity.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures

Similar articles
-
MOTS-c: A promising mitochondrial-derived peptide for therapeutic exploitation.Front Endocrinol (Lausanne). 2023 Jan 25;14:1120533. doi: 10.3389/fendo.2023.1120533. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36761202 Free PMC article. Review.
-
Acute endurance exercise stimulates circulating levels of mitochondrial-derived peptides in humans.J Appl Physiol (1985). 2021 Sep 1;131(3):1035-1042. doi: 10.1152/japplphysiol.00706.2019. Epub 2021 Aug 5. J Appl Physiol (1985). 2021. PMID: 34351816 Clinical Trial.
-
Mitochondrial-derived peptides and exercise.Biochim Biophys Acta Gen Subj. 2021 Dec;1865(12):130011. doi: 10.1016/j.bbagen.2021.130011. Epub 2021 Sep 11. Biochim Biophys Acta Gen Subj. 2021. PMID: 34520826 Review.
-
A Mitochondrial Encoded Messenger at the Nucleus.Cells. 2018 Aug 13;7(8):105. doi: 10.3390/cells7080105. Cells. 2018. PMID: 30104535 Free PMC article.
-
Mitochondrial-derived peptides in energy metabolism.Am J Physiol Endocrinol Metab. 2020 Oct 1;319(4):E659-E666. doi: 10.1152/ajpendo.00249.2020. Epub 2020 Aug 10. Am J Physiol Endocrinol Metab. 2020. PMID: 32776825 Free PMC article. Review.
Cited by
-
MOTS-c Serum Concentration Positively Correlates with Lower-Body Muscle Strength and Is Not Related to Maximal Oxygen Uptake-A Preliminary Study.Int J Mol Sci. 2023 Oct 6;24(19):14951. doi: 10.3390/ijms241914951. Int J Mol Sci. 2023. PMID: 37834399 Free PMC article.
-
Sedentary Lifestyles and a Hypercaloric Diets During Middle Age, are Binomial Conducive to Fatal Progression, That is Counteracted by the Hormetic Treatment of Exercise, Metformin, and Tert-Butyl Hydroquinone: An Analysis of Female Middle-Aged Rat Liver Mitochondria.Dose Response. 2024 Oct 10;22(4):15593258241272619. doi: 10.1177/15593258241272619. eCollection 2024 Oct-Dec. Dose Response. 2024. PMID: 39399210 Free PMC article.
-
MOTS-c: A promising mitochondrial-derived peptide for therapeutic exploitation.Front Endocrinol (Lausanne). 2023 Jan 25;14:1120533. doi: 10.3389/fendo.2023.1120533. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 36761202 Free PMC article. Review.
-
Mitochondrial-derived peptides: Antidiabetic functions and evolutionary perspectives.Peptides. 2024 Feb;172:171147. doi: 10.1016/j.peptides.2023.171147. Epub 2023 Dec 29. Peptides. 2024. PMID: 38160808 Free PMC article. Review.
-
Mitochondria-derived peptide is an effective target for treating streptozotocin induced painful diabetic neuropathy through induction of activated protein kinase/peroxisome proliferator-activated receptor gamma coactivator 1alpha -mediated mitochondrial biogenesis.Mol Pain. 2024 Jan-Dec;20:17448069241252654. doi: 10.1177/17448069241252654. Mol Pain. 2024. PMID: 38658141 Free PMC article.
References
-
- Celik O, Yildiz BO. Obesity and physical exercise. Minerva Endocrinol (Torino) 2021;46:131–44. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

