Oligoprogression in Metastatic, Castrate-Resistant Prostate Cancer-Prevalence and Current Clinical Practice
- PMID: 35656509
- PMCID: PMC9152030
- DOI: 10.3389/fonc.2022.862995
Oligoprogression in Metastatic, Castrate-Resistant Prostate Cancer-Prevalence and Current Clinical Practice
Abstract
Aims: Oligoprogression is poorly defined in current literature. Little is known about the natural history and significance of oligoprogression in patients with hormone-resistant prostate cancer on abiraterone or enzalutamide treatment [termed androgen receptor-targeted therapy (ARTT)]. The aim of this study was to determine the prevalence of oligoprogression, describe the characteristics of oligoprogression in a cohort of patients from a single center, and identify the number of patients potentially treatable with stereotactic body radiotherapy (SBRT).
Methods: Castration-resistant prostate cancer (CRPC) patients who radiologically progressed while on ARTT were included. Patients with oligoprogressive disease (OPD) (≤3 lesions) on any imaging were identified in a retrospective analysis of electronic patient records. Kaplan-Meier method and log-rank test were used to calculate progression-free and overall survival.
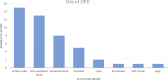
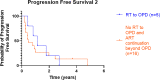
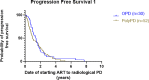
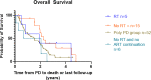
Results: A total of 102 patients with metastatic CRPC on ARTT were included. Thirty (29%) patients presented with oligoprogression (46 lesions in total); 21 (21% of total) patients had lesions suitable for SBRT. The majority of lesions were in the bone (21, 46%) or lymph nodes (15, 33%). Patients with oligoprogression while on ARTT had a significantly better prostate-specific antigen (PSA) response on commencing ARTT as compared to patients who later developed polyprogression. However, PSA doubling time immediately prior to progression did not predict OPD. Median progression-free survival to oligoprogression versus polyprogression was 16.8 vs. 11.7 months. Time to further progression after oligoprogression was 13.6 months in those treated with radiotherapy (RT) for oligoprogression vs. 5.7 months in those treated with the continuation of ARTT alone.
Conclusions: In this study, nearly a third of patients on ARTT for CRPC were found to have OPD. OPD patients had a better PSA response on ART and a longer duration on ARTT before developing OPD as compared to those developing polyprogressive disease (Poly-PD). The majority of patients (70%) with OPD had lesions suitable for SBRT treatment. Prospective randomized control trials are needed to establish if there is a survival benefit of SBRT in oligoprogressive prostate cancer and to determine predictive indicators.
Keywords: Androgen receptor targeted therapy; Oligoprogressive disease (OPD); abiraterone; castrate resistant prostate cancer; enzalutamide; oligoprogression; stereotactic body radiotherapy.
Copyright © 2022 Patel, Tunariu, Levine, de Bono, Eeles, Khoo, Murray, Parker, Pathmanathan, Reid, van As and Tree.
Conflict of interest statement
AT has received research funding and/or travel funding from Elekta, Varian, and Accuray, and honoraria/travel budget support from Janssen and historically from Astellas. AP has received research funding from Elekta and honoraria from Elekta and Janssen. JB has served on advisory boards and received fees from many companies including Amgen, Astra Zeneca, Astellas, Bayer, Bioxcel Therapeutics, Boehringer Ingelheim, Cellcentric, Daiichi, Eisai, Genentech/Roche, Genmab, GSK, Harpoon, ImCheck Therapeutics, Janssen, Merck Serono, Merck Sharp & Dohme, Menarini/Silicon Biosystems, Orion, Pfizer, Qiagen, Sanofi Aventis, Sierra Oncology, Taiho, Terumo, and Vertex Pharmaceuticals. He is an employee of ICR and has received funding or other support for his research work from AZ, Astellas, Bayer, Cellcentric, Daiichi, Genentech, Genmab, GSK, Janssen, Merck Serono, MSD, Menarini/Silicon Biosystems, Orion, Sanofi Aventis, Sierra Oncology, Taiho, Pfizer, and Vertex, which has a commercial interest in abiraterone, PARP inhibition in DNA repair defective cancers, and PI3K/AKT pathway inhibitors (no personal income). JB was named as an inventor, with no financial interest for patent 8,822,438, submitted by Janssen, that covers the use of abiraterone acetate with corticosteroids. He has been the CI/PI of many industry-sponsored clinical trials. JB is a National Institute for Health Research (NIHR) Senior Investigator. CP served on Bayer in the education steering committee on January 21, 2020. He received payment from ICR for the podcast on mCRPC, July 2021; speaker fees from Janssen for Summit on Feb 2020, lecture on Nov 2020, and Ad board on March 2020; fees from Clarity Pharmaceuticals for Ad board on April 2020; fees from Myovant for Ad board on October 2020; fees from ITM Radiopharma for Ad board on October 2021; payment from AAA to ICR for Ad board on Dec 2021. RE declares receiving honorarium as a speaker at GU-ASCO Meeting 2016 and support from Janssen, as a speaker at the Royal Marsden NHS Foundation Trust 2017. She has received an honorarium as a speaker at the University of Chicago in 2018 and Bayer and Ipsen honoraria at the ESMO meeting in 2019, and she is a member of the external expert committee of AstraZeneca UK Limited. PP received an honorarium for educational cases for PinPoint Case Platform, Mirrors of Medicine, and research post funded by the Royal Marsden/Institute of Cancer Research Biomedical Research Centre and Prostate Cancer UK. N.J.V.A. declares consultant honorarium from Accuray and Research funds from Accuray. AR has received honoraria and travel support from Janssen, Astellas and AZD. VK reports honoraria for speakers bureaus, personal fees and non-financial support from Accuray, Astellas, Bayer, Boston Scientific, and Janssen. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Stereotactic Body Radiotherapy for Oligoprogression in Castration-Resistant Prostate Cancer: Early Toxicity Analysis of the TRAP Trial.Clin Oncol (R Coll Radiol). 2024 Sep;36(9):585-592. doi: 10.1016/j.clon.2024.06.047. Epub 2024 Jun 18. Clin Oncol (R Coll Radiol). 2024. PMID: 39004535 Clinical Trial.
-
Androgen Receptor Targeted Therapy + Radiotherapy in Metastatic Castration Resistant Prostate Cancer.Front Oncol. 2021 Sep 23;11:695136. doi: 10.3389/fonc.2021.695136. eCollection 2021. Front Oncol. 2021. PMID: 34631527 Free PMC article.
-
Radiotherapy in metastatic castration resistant prostate cancer patients with oligo-progression during abiraterone-enzalutamide treatment: a mono-institutional experience.Radiat Oncol. 2019 Nov 14;14(1):205. doi: 10.1186/s13014-019-1414-x. Radiat Oncol. 2019. PMID: 31727093 Free PMC article.
-
The current landscape of stereotactic body radiation therapy for metastatic castration-resistant prostate cancer.Prostate Cancer Prostatic Dis. 2024 Jun 19. doi: 10.1038/s41391-024-00862-8. Online ahead of print. Prostate Cancer Prostatic Dis. 2024. PMID: 38898265 Review.
-
EAU guidelines on prostate cancer. Part II: Treatment of advanced, relapsing, and castration-resistant prostate cancer.Eur Urol. 2014 Feb;65(2):467-79. doi: 10.1016/j.eururo.2013.11.002. Epub 2013 Nov 12. Eur Urol. 2014. PMID: 24321502 Review.
Cited by
-
Exploring the mechanism of action of Sparganii Rhizoma-Curcumae Rhizoma for in treating castration-resistant prostate cancer: a network-based pharmacology and experimental validation study.Sci Rep. 2024 Feb 7;14(1):3099. doi: 10.1038/s41598-024-53699-5. Sci Rep. 2024. PMID: 38326539 Free PMC article.
-
Treatment response assessment in mCRPC: is PSMA-PET/CT going to take the lead?Ther Adv Med Oncol. 2024 Oct 7;16:17588359241258367. doi: 10.1177/17588359241258367. eCollection 2024. Ther Adv Med Oncol. 2024. PMID: 39386313 Free PMC article. Review.
References
-
- CRUK . Cancer Research UK (2020). Available at: https://www.cancerresearchuk.org/about-cancer/prostate-cancer/risks-causes.
-
- Ng K, Wilson P, Mutsvangwa K, Hounsome L, Shamash J. Overall Survival of Black and White Men With Metastatic Castration-Resistant Prostate Cancer (mCRPC): A 20-Year Retrospective Analysis in the Largest Healthcare Trust in England. Prostate Cancer Prostatic Dis (2021) 24:718–24. doi: 10.1038/s41391-020-00316-x - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

