Faulty autolysosome acidification in Alzheimer's disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques
- PMID: 35654956
- PMCID: PMC9174056
- DOI: 10.1038/s41593-022-01084-8
Faulty autolysosome acidification in Alzheimer's disease mouse models induces autophagic build-up of Aβ in neurons, yielding senile plaques
Abstract
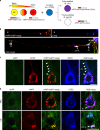
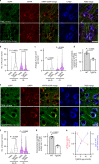
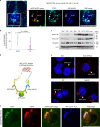
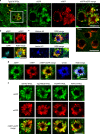
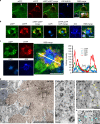
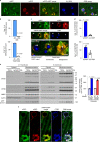
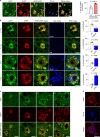
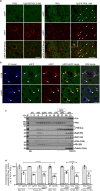
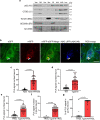
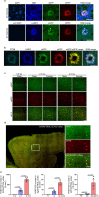
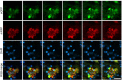
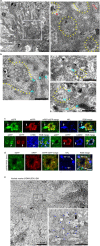
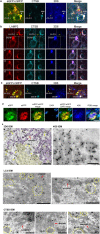
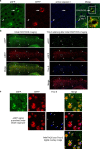
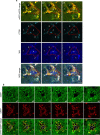
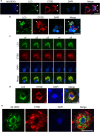
Autophagy is markedly impaired in Alzheimer's disease (AD). Here we reveal unique autophagy dysregulation within neurons in five AD mouse models in vivo and identify its basis using a neuron-specific transgenic mRFP-eGFP-LC3 probe of autophagy and pH, multiplex confocal imaging and correlative light electron microscopy. Autolysosome acidification declines in neurons well before extracellular amyloid deposition, associated with markedly lowered vATPase activity and build-up of Aβ/APP-βCTF selectively within enlarged de-acidified autolysosomes. In more compromised yet still intact neurons, profuse Aβ-positive autophagic vacuoles (AVs) pack into large membrane blebs forming flower-like perikaryal rosettes. This unique pattern, termed PANTHOS (poisonous anthos (flower)), is also present in AD brains. Additional AVs coalesce into peri-nuclear networks of membrane tubules where fibrillar β-amyloid accumulates intraluminally. Lysosomal membrane permeabilization, cathepsin release and lysosomal cell death ensue, accompanied by microglial invasion. Quantitative analyses confirm that individual neurons exhibiting PANTHOS are the principal source of senile plaques in amyloid precursor protein AD models.
© 2022. The Author(s).
Conflict of interest statement
All authors declare no competing interests.
Figures

Comment in
-
Opening the box of PANTHORA in Alzheimer's disease.Signal Transduct Target Ther. 2022 Oct 2;7(1):344. doi: 10.1038/s41392-022-01204-7. Signal Transduct Target Ther. 2022. PMID: 36184585 Free PMC article. No abstract available.
Similar articles
-
Autolysosomal acidification failure as a primary driver of Alzheimer disease pathogenesis.Autophagy. 2022 Nov;18(11):2763-2764. doi: 10.1080/15548627.2022.2110729. Epub 2022 Aug 23. Autophagy. 2022. PMID: 35947489 Free PMC article.
-
Neuronal-Targeted TFEB Accelerates Lysosomal Degradation of APP, Reducing Aβ Generation and Amyloid Plaque Pathogenesis.J Neurosci. 2015 Sep 2;35(35):12137-51. doi: 10.1523/JNEUROSCI.0705-15.2015. J Neurosci. 2015. PMID: 26338325 Free PMC article.
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Autophagy-lysosomal-associated neuronal death in neurodegenerative disease.Acta Neuropathol. 2024 Sep 11;148(1):42. doi: 10.1007/s00401-024-02799-7. Acta Neuropathol. 2024. PMID: 39259382 Review.
-
Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death.Metab Brain Dis. 2020 Jan;35(1):11-30. doi: 10.1007/s11011-019-00516-y. Epub 2019 Dec 6. Metab Brain Dis. 2020. PMID: 31811496 Review.
Cited by
-
Astrocytes Excessively Engulf Synapses in a Mouse Model of Alzheimer's Disease.Int J Mol Sci. 2024 Jan 18;25(2):1160. doi: 10.3390/ijms25021160. Int J Mol Sci. 2024. PMID: 38256233 Free PMC article.
-
Targeting autophagy in Alzheimer's disease: Animal models and mechanisms.Zool Res. 2023 Nov 18;44(6):1132-1145. doi: 10.24272/j.issn.2095-8137.2023.294. Zool Res. 2023. PMID: 37963840 Free PMC article. Review.
-
Neuroprotective Effects of Agri-Food By-Products Rich in Phenolic Compounds.Nutrients. 2023 Jan 14;15(2):449. doi: 10.3390/nu15020449. Nutrients. 2023. PMID: 36678322 Free PMC article. Review.
-
A Mouse Systems Genetics Approach Reveals Common and Uncommon Genetic Modifiers of Hepatic Lysosomal Enzyme Activities and Glycosphingolipids.Int J Mol Sci. 2023 Mar 3;24(5):4915. doi: 10.3390/ijms24054915. Int J Mol Sci. 2023. PMID: 36902345 Free PMC article.
-
Elevated levels of tripeptidyl peptidase 1 do not ameliorate pathogenesis in a mouse model of Alzheimer disease.Neurobiol Aging. 2022 Oct;118:106-107. doi: 10.1016/j.neurobiolaging.2022.06.012. Epub 2022 Jul 7. Neurobiol Aging. 2022. PMID: 35914472 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

