TGF-β1 mediates pathologic changes of secondary lymphedema by promoting fibrosis and inflammation
- PMID: 35652284
- PMCID: PMC9160979
- DOI: 10.1002/ctm2.758
TGF-β1 mediates pathologic changes of secondary lymphedema by promoting fibrosis and inflammation
Abstract
Background: Secondary lymphedema is a common complication of cancer treatment, and previous studies have shown that the expression of transforming growth factor-beta 1 (TGF-β1), a pro-fibrotic and anti-lymphangiogenic growth factor, is increased in this disease. Inhibition of TGF-β1 decreases the severity of the disease in mouse models; however, the mechanisms that regulate this improvement remain unknown.
Methods: Expression of TGF-β1 and extracellular matrix molecules (ECM) was assessed in biopsy specimens from patients with unilateral breast cancer-related lymphedema (BCRL). The effects of TGF-β1 inhibition using neutralizing antibodies or a topical formulation of pirfenidone (PFD) were analyzed in mouse models of lymphedema. We also assessed the direct effects of TGF-β1 on lymphatic endothelial cells (LECs) using transgenic mice that expressed a dominant-negative TGF-β receptor selectively on LECs (LECDN-RII ).
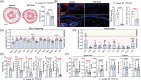
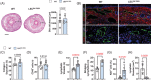
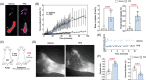
Results: The expression of TGF-β1 and ECM molecules is significantly increased in BCRL skin biopsies. Inhibition of TGF-β1 in mouse models of lymphedema using neutralizing antibodies or with topical PFD decreased ECM deposition, increased the formation of collateral lymphatics, and inhibited infiltration of T cells. In vitro studies showed that TGF-β1 in lymphedematous tissues increases fibroblast, lymphatic endothelial cell (LEC), and lymphatic smooth muscle cell stiffness. Knockdown of TGF-β1 responsiveness in LECDN-RII resulted in increased lymphangiogenesis and collateral lymphatic formation; however, ECM deposition and fibrosis persisted, and the severity of lymphedema was indistinguishable from controls.
Conclusions: Our results show that TGF-β1 is an essential regulator of ECM deposition in secondary lymphedema and that inhibition of this response is a promising means of treating lymphedema.
Keywords: TGF-β; fibrosis; inflammation; pirfenidone.
© 2022 The Authors. Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
Dr. Mehrara is an advisor to PureTech Corporation and recipient of an investigator‐initiated award from PureTech and Regeneron Corp.
Figures




Similar articles
-
TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair.Am J Physiol Heart Circ Physiol. 2008 Nov;295(5):H2113-27. doi: 10.1152/ajpheart.00879.2008. Epub 2008 Oct 10. Am J Physiol Heart Circ Physiol. 2008. PMID: 18849330
-
Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair.Am J Pathol. 2010 Dec;177(6):3202-14. doi: 10.2353/ajpath.2010.100594. Epub 2010 Nov 5. Am J Pathol. 2010. PMID: 21056998 Free PMC article.
-
Topical captopril: a promising treatment for secondary lymphedema.Transl Res. 2023 Jul;257:43-53. doi: 10.1016/j.trsl.2023.01.005. Epub 2023 Feb 1. Transl Res. 2023. PMID: 36736951 Free PMC article.
-
Lymphatic endothelial cells, lymphedematous lymphangiogenesis, and molecular control of edema formation.Lymphat Res Biol. 2008;6(3-4):123-37. doi: 10.1089/lrb.2008.1005. Lymphat Res Biol. 2008. PMID: 19093784 Review.
-
Role of Platelet-Derived Transforming Growth Factor-β1 and Reactive Oxygen Species in Radiation-Induced Organ Fibrosis.Antioxid Redox Signal. 2017 Nov 1;27(13):977-988. doi: 10.1089/ars.2017.7064. Epub 2017 Jul 5. Antioxid Redox Signal. 2017. PMID: 28562065 Free PMC article. Review.
Cited by
-
Decoding tumor-fibrosis interplay: mechanisms, impact on progression, and innovative therapeutic strategies.Front Pharmacol. 2024 Oct 23;15:1491400. doi: 10.3389/fphar.2024.1491400. eCollection 2024. Front Pharmacol. 2024. PMID: 39534084 Free PMC article. Review.
-
Molecular pathophysiology of secondary lymphedema.Front Cell Dev Biol. 2024 Jul 8;12:1363811. doi: 10.3389/fcell.2024.1363811. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39045461 Free PMC article. Review.
-
[Research advances on stem cell-based treatments in animal studies and clinical trials of lymphedema].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024 Jan 15;38(1):99-106. doi: 10.7507/1002-1892.202309045. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024. PMID: 38225848 Free PMC article. Chinese.
-
TGF-β signaling in lymphatic vascular vessel formation and maintenance.Front Physiol. 2022 Dec 15;13:1081376. doi: 10.3389/fphys.2022.1081376. eCollection 2022. Front Physiol. 2022. PMID: 36589453 Free PMC article. Review.
-
Crosstalk of ubiquitin system and non-coding RNA in fibrosis.Int J Biol Sci. 2024 Jul 8;20(10):3802-3822. doi: 10.7150/ijbs.93644. eCollection 2024. Int J Biol Sci. 2024. PMID: 39113708 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
