RNA-Based Therapy for Cryptosporidium parvum Infection: Proof-of-Concept Studies
- PMID: 35647663
- PMCID: PMC9302151
- DOI: 10.1128/iai.00196-22
RNA-Based Therapy for Cryptosporidium parvum Infection: Proof-of-Concept Studies
Abstract
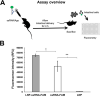
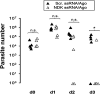
Cryptosporidium is a leading cause of moderate-to-severe diarrhea in children, which is one of the major causes of death in children under 5 years old. Nitazoxanide is the only FDA-approved treatment for cryptosporidiosis. However, it has limited efficacy in immunosuppressed patients and malnourished children. Therefore, it is urgent to develop novel therapies against this parasite. RNA interference-mediated therapies are emerging as novel approaches for the treatment of infectious diseases. We have developed a novel method to silence essential genes in Cryptosporidium using single-stranded RNA (ssRNA)/Argonaute (Ago) complexes. In this work we conducted proof-of-concept studies to test the anticryptosporidial activity of these complexes by silencing Cryptosporidium parvum nucleoside diphosphate kinase (NDK) using in vitro and in vivo models. We demonstrated that a 3-day treatment with anti-sense NDK ssRNA/Ago decreased parasite burden by ~98% on infected cells. In vivo studies showed that ssRNA/Ago complexes encapsulated in lipid nanoparticles can be delivered onto intestinal epithelial cells of mice treated orally. In addition a cryptosporidiosis-mouse model showed that treatment with NDK ssRNA/Ago complexes reduced oocyst shedding in 4/5 SCID/beige mice during the acute phase of the infection. Our findings highlight the potential use of antisense RNA-based therapy as an alternative approach to cryptosporidiosis treatment.
Keywords: Argonaute; Cryptosporidium; NDK; cryptosporidiosis; gene silencing; siRNA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Preassembled Single-Stranded RNA-Argonaute Complexes: A Novel Method to Silence Genes in Cryptosporidium.J Infect Dis. 2016 Apr 15;213(8):1307-14. doi: 10.1093/infdis/jiv588. Epub 2015 Dec 11. J Infect Dis. 2016. PMID: 26656125 Free PMC article.
-
A high-throughput phenotypic screen identifies clofazimine as a potential treatment for cryptosporidiosis.PLoS Negl Trop Dis. 2017 Feb 3;11(2):e0005373. doi: 10.1371/journal.pntd.0005373. eCollection 2017 Feb. PLoS Negl Trop Dis. 2017. PMID: 28158186 Free PMC article.
-
Systematic gene silencing identified Cryptosporidium nucleoside diphosphate kinase and other molecules as targets for suppression of parasite proliferation in human intestinal cells.Sci Rep. 2019 Aug 21;9(1):12153. doi: 10.1038/s41598-019-48544-z. Sci Rep. 2019. PMID: 31434931 Free PMC article.
-
Cryptosporidiosis Drug Discovery: Opportunities and Challenges.ACS Infect Dis. 2016 Aug 12;2(8):530-7. doi: 10.1021/acsinfecdis.6b00094. Epub 2016 Jul 22. ACS Infect Dis. 2016. PMID: 27626293 Review.
-
Novel treatment strategies and drugs in development for cryptosporidiosis.Expert Rev Anti Infect Ther. 2018 Aug;16(8):655-661. doi: 10.1080/14787210.2018.1500457. Epub 2018 Jul 17. Expert Rev Anti Infect Ther. 2018. PMID: 30003818 Review.
Cited by
-
Cryptosporidium Genomics - Current Understanding, Advances, and Applications.Curr Trop Med Rep. 2024;11(2):92-103. doi: 10.1007/s40475-024-00318-y. Epub 2024 Mar 23. Curr Trop Med Rep. 2024. PMID: 38813571 Free PMC article. Review.
-
RNA-Based Vaccines and Therapeutics Against Intracellular Pathogens.Methods Mol Biol. 2024;2813:321-370. doi: 10.1007/978-1-0716-3890-3_21. Methods Mol Biol. 2024. PMID: 38888787 Review.
References
-
- Checkley W, White AC, Jr, Jaganath D, Arrowood MJ, Chalmers RM, Chen XM, Fayer R, Griffiths JK, Guerrant RL, Hedstrom L, Huston CD, Kotloff KL, Kang G, Mead JR, Miller M, Petri WA, Jr, Priest JW, Roos DS, Striepen B, Thompson RC, Ward HD, Van Voorhis WA, Xiao L, Zhu G, Houpt ER. 2015. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect Dis 15:85–94. 10.1016/S1473-3099(14)70772-8. - DOI - PMC - PubMed
-
- Khalil IA, Troeger C, Rao PC, Blacker BF, Brown A, Brewer TG, Colombara DV, De Hostos EL, Engmann C, Guerrant RL, Haque R, Houpt ER, Kang G, Korpe PS, Kotloff KL, Lima AAM, Petri WA, Jr, Platts-Mills JA, Shoultz DA, Forouzanfar MH, Hay SI, Reiner RC, Jr, Mokdad AH. 2018. Morbidity, mortality, and long-term consequences associated with diarrhoea from Cryptosporidium infection in children younger than 5 years: a meta-analyses study. Lancet Glob Health 6:e758–e768. 10.1016/S2214-109X(18)30283-3. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

