Bladder Oxidative Stress and HMGB1 Release Contribute to PAR4-Mediated Bladder Pain in Mice
- PMID: 35645739
- PMCID: PMC9135998
- DOI: 10.3389/fnsys.2022.882493
Bladder Oxidative Stress and HMGB1 Release Contribute to PAR4-Mediated Bladder Pain in Mice
Abstract
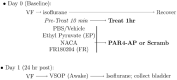
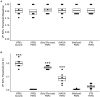
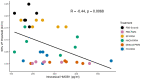
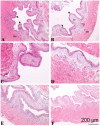
Activation of intravesical PAR4 receptors leads to bladder hyperalgesia (BHA) through release of urothelial macrophage migration inhibitory factor (MIF) and urothelial high mobility group box-1 (HMGB1). MIF deficiency and/or MIF antagonism at the bladder block BHA in mice yet the mechanisms are not clear. Since oxidative stress and ERK phosphorylation are involved in MIF signaling we hypothesized that oxidative stress and/or ERK signaling, activated by MIF release, promote intravesical HMGB1 release to induce BHA. We induced BHA by intravesical PAR4 infusion in female C57BL/6 mice. Mechanical sensitivity was evaluated by measuring abdominal von Frey (VF) 50% thresholds before (baseline) and 24 h post-infusion. Intravesical pre-treatment (10 min infusion prior to PAR4) with N-acetylcysteine amide (NACA; reactive-oxygen species scavenger; 3 mg in 50 μl), FR180204 (selective ERK1/2 inhibitor; 200 μg in 50 μl), ethyl pyruvate (EP; HMGB1 release inhibitor; 600 μg in 50 μl), or diluent controls (50 μl) tested the effects of pre-treatment on PAR4-induced BHA. Intravesical fluid was collected after each treatment and HMGB1 concentration was measured using ELISA. Awake micturition parameters (volume and frequency) were assessed at the end of the experiments. Bladders were collected and examined for histological signs of edema and inflammation. Pre-treatment with PBS followed by PAR4 induced BHA in mice but PBS followed by scrambled peptide did not. Pre-treatment with NACA or EP partially blocked PAR4-induced BHA while FR180204 had no effect. A significant correlation between intravesical HMGB1 levels and 50% VF thresholds was observed. All PAR4 treated groups had increased levels of HMGB1 in the intravesical fluid compared to PBS-Scrambled group although not statistically significant. No significant effects were noted on awake micturition volume, micturition frequency or histological evidence of bladder edema or inflammation. Our results show that intravesical antagonism of bladder reactive-oxygen species accumulation was effective in reducing PAR4-induced bladder pain. The correlation between intravesical levels of HMGB1 and bladder pain indicates that released HMGB1 is pivotal to bladder pain. Thus, modulating events in the MIF signaling cascade triggered by PAR4 activation (including bladder oxidative stress and HMGB1 release) warrant further investigation as possible therapeutic strategies.
Keywords: HMGB1; MIF; PAR4; ROS; bladder pain.
Copyright © 2022 Ye, Ma, Mahmood, Meyer-Siegler, Leng, Bucala and Vera.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
Urothelial Oxidative Stress and ERK Activation Mediate HMGB1-Induced Bladder Pain.Cells. 2023 May 22;12(10):1440. doi: 10.3390/cells12101440. Cells. 2023. PMID: 37408274 Free PMC article.
-
Macrophage migration inhibitory factor mediates protease-activated receptor 4-induced bladder pain through urothelial high mobility group box 1.Physiol Rep. 2017 Dec;5(24):e13549. doi: 10.14814/phy2.13549. Physiol Rep. 2017. PMID: 29263120 Free PMC article.
-
Modulation of persistent bladder pain in mice: The role of macrophage migration inhibitory factor, high mobility group box-1, and downstream signaling pathways.Bladder (San Franc). 2024 Oct 28;11(2):e21200011. doi: 10.14440/bladder.2024.0015. eCollection 2024. Bladder (San Franc). 2024. PMID: 39539469 Free PMC article.
-
Intravesical treatments for painful bladder syndrome/ interstitial cystitis.Cochrane Database Syst Rev. 2007 Oct 17;(4):CD006113. doi: 10.1002/14651858.CD006113.pub2. Cochrane Database Syst Rev. 2007. PMID: 17943887 Review.
-
High-mobility group box 1, oxidative stress, and disease.Antioxid Redox Signal. 2011 Apr 1;14(7):1315-35. doi: 10.1089/ars.2010.3356. Antioxid Redox Signal. 2011. PMID: 20969478 Free PMC article. Review.
Cited by
-
Mechanisms of oxidative stress in interstitial cystitis/bladder pain syndrome.Nat Rev Urol. 2024 Jul;21(7):433-449. doi: 10.1038/s41585-023-00850-y. Epub 2024 Feb 7. Nat Rev Urol. 2024. PMID: 38326514 Review.
-
Urothelial Oxidative Stress and ERK Activation Mediate HMGB1-Induced Bladder Pain.Cells. 2023 May 22;12(10):1440. doi: 10.3390/cells12101440. Cells. 2023. PMID: 37408274 Free PMC article.
-
Cannabidiol as a Promising Therapeutic Option in IC/BPS: In Vitro Evaluation of Its Protective Effects against Inflammation and Oxidative Stress.Int J Mol Sci. 2023 Mar 6;24(5):5055. doi: 10.3390/ijms24055055. Int J Mol Sci. 2023. PMID: 36902479 Free PMC article.
-
Lumbosacral spinal proteomic changes during PAR4-induced persistent bladder pain.Neurosci Lett. 2024 Jan 1;818:137563. doi: 10.1016/j.neulet.2023.137563. Epub 2023 Nov 28. Neurosci Lett. 2024. PMID: 38036085 Free PMC article.
-
Chronic E. Coli Drug-Resistant Cystitis Treated with a Sequence of Modulated Extremely Low-Frequency Electromagnetic Fields: A Randomized Study of 148 Cases.J Clin Med. 2024 Apr 30;13(9):2639. doi: 10.3390/jcm13092639. J Clin Med. 2024. PMID: 38731168 Free PMC article.
References
-
- Alam A., Haldar S., Thulasiram H. V., Kumar R., Goyal M., Iqbal M. S., et al. . (2012). Novel anti-inflammatory activity of epoxyazadiradione against macrophage migration inhibitory factor: inhibition of tautomerase and proinflammatory activities of macrophage migration inhibitory factor. J. Biol. Chem. 287, 24844–24861. 10.1074/jbc.M112.341321 - DOI - PMC - PubMed
-
- Alexander J. K., Cox G. M., Tian J.-B., Zha A. M., Wei P., Kigerl K. A., et al. . (2012). Macrophage migration inhibitory factor (MIF) is essential for inflammatory and neuropathic pain and enhances pain in response to stress. Exp. Neurol. 236, 351–362. 10.1016/j.expneurol.2012.04.018 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

