Timing of adjuvant chemotherapy initiation and mortality among colon cancer patients at a safety-net health system
- PMID: 35641921
- PMCID: PMC9158363
- DOI: 10.1186/s12885-022-09688-w
Timing of adjuvant chemotherapy initiation and mortality among colon cancer patients at a safety-net health system
Abstract
Background: Prior studies reported survival benefits from early initiation of adjuvant chemotherapy for stage III colon cancer, but this evidence was derived from studies that may be sensitive to time-related biases. Therefore, we aimed to estimate the effect of initiating adjuvant chemotherapy ≤8 or ≤ 12 weeks on overall and disease-free survival among stage III colon cancer patients using a study design that helps address time-related biases.
Methods: We used institutional registry data from JPS Oncology and Infusion Center, a Comprehensive Community Cancer Program. Eligible patients were adults aged < 80 years, diagnosed with first primary stage III colon cancer between 2011 and 2017, and received surgical resection with curative intent. We emulated a target trial with sequential eligibility. We subsequently pooled the trials and estimated risk ratios (RRs) along with 95% confidence limits (CL) for all-cause mortality and recurrence or death at 5-years between initiators and non-initiators of adjuvant chemotherapy ≤8 or ≤ 12 weeks using pseudo-observations and a marginal structural model with stabilized inverse probability of treatment weights.
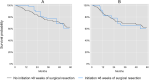
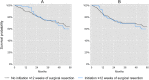
Results: Our study population comprised 222 (for assessing initiation ≤8 weeks) and 310 (for assessing initiation ≤12 weeks) observations, of whom the majority were racial/ethnic minorities (64-65%), or uninsured with or without enrollment in our hospital-based medical assistance program (68-71%). Initiation of adjuvant chemotherapy ≤8 weeks of surgical resection did not improve overall survival (RR for all-cause mortality = 1.04, 95% CL: 0.57, 1.92) or disease-free survival (RR for recurrence or death = 1.07, 95% CL: 0.61, 1.88). The results were similar for initiation of adjuvant chemotherapy ≤12 weeks of surgical resection.
Conclusions: Our results suggest that the overall and disease-free survival benefits of initiating adjuvant chemotherapy ≤8 or ≤ 12 weeks of surgical resection may be overestimated in prior studies, which may be attributable to time-related biases. Nevertheless, our estimates were imprecise and differences in population characteristics are an alternate explanation. Additional studies that address time-related biases are needed to clarify our findings.
Keywords: Adjuvant chemotherapy; Disease-free survival; Mortality; Prognosis; Quality of care; Timing; colon cancer.
© 2022. The Author(s).
Conflict of interest statement
The authors have no financial or non-financial competing interests to disclose.
Figures
Similar articles
-
Timing of adjuvant chemotherapy and its relation to survival among patients with stage III colon cancer.Eur J Cancer. 2015 Nov;51(17):2553-61. doi: 10.1016/j.ejca.2015.08.016. Epub 2015 Sep 7. Eur J Cancer. 2015. PMID: 26360411
-
Determining the Optimal Timing for Initiation of Adjuvant Chemotherapy After Resection for Stage II and III Colon Cancer.Dis Colon Rectum. 2016 Feb;59(2):87-93. doi: 10.1097/DCR.0000000000000518. Dis Colon Rectum. 2016. PMID: 26734965
-
Timing of adjuvant chemotherapy initiation after surgery for stage III colon cancer.Cancer. 2006 Dec 1;107(11):2581-8. doi: 10.1002/cncr.22316. Cancer. 2006. PMID: 17078055
-
Adjuvant Chemotherapy for Stage II and III Colon Cancer Following Complete Resection: A Cancer Care Ontario Systematic Review.Clin Oncol (R Coll Radiol). 2017 Jul;29(7):459-465. doi: 10.1016/j.clon.2017.03.001. Epub 2017 Mar 22. Clin Oncol (R Coll Radiol). 2017. PMID: 28341242 Review.
-
Adjuvant therapy for stage II colon cancer: a systematic review from the Cancer Care Ontario Program in evidence-based care's gastrointestinal cancer disease site group.J Clin Oncol. 2004 Aug 15;22(16):3395-407. doi: 10.1200/JCO.2004.03.087. Epub 2004 Jun 15. J Clin Oncol. 2004. PMID: 15199087 Review.
Cited by
-
Reporting of Observational Studies Explicitly Aiming to Emulate Randomized Trials: A Systematic Review.JAMA Netw Open. 2023 Sep 5;6(9):e2336023. doi: 10.1001/jamanetworkopen.2023.36023. JAMA Netw Open. 2023. PMID: 37755828 Free PMC article.
-
Oncologic outcomes for robotic versus laparoscopic colectomy for colon cancer: an ACS-NSQIP analysis.J Robot Surg. 2024 Sep 17;18(1):341. doi: 10.1007/s11701-024-02097-0. J Robot Surg. 2024. PMID: 39287882
References
-
- Laurie JA, Moertel CG, Fleming TR, Wieand HS, Leigh JE, Rubin J, McCormack GW, Gerstner JB, Krook JE, Malliard J, et al. Surgical adjuvant therapy of large-bowel carcinoma: an evaluation of levamisole and the combination of levamisole and fluorouracil. The north central Cancer treatment group and the Mayo Clinic. J Clin Oncol. 1989;7(10):1447–1456. doi: 10.1200/JCO.1989.7.10.1447. - DOI - PubMed
-
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen CM, Ungerleider JS, Emerson WA, Tormey DC, Glick JH, et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon carcinoma: a final report. Ann Intern Med. 1995;122(5):321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources

