Chemotherapy Resistance in B-ALL with Cryptic NUP214-ABL1 Is Amenable to Kinase Inhibition and Immunotherapy
- PMID: 35641210
- PMCID: PMC8895729
- DOI: 10.1093/oncolo/oyab052
Chemotherapy Resistance in B-ALL with Cryptic NUP214-ABL1 Is Amenable to Kinase Inhibition and Immunotherapy
Abstract
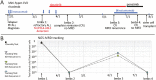
BCR-ABL1 kinase inhibitors have improved the prognosis of Philadelphia-chromosome-positive (Ph+)-acute lymphoblastic leukemia (ALL). Ph-like (or BCR-ABL1-like) ALL does not express BCR-ABL1 but commonly harbors other genomic alterations of signaling molecules that may be amenable to therapy. Here, we report a case with a NUP214-ABL1 fusion detected at relapse by multiplexed, targeted RNA sequencing. It had escaped conventional molecular work-up at diagnosis, including cytogenetic analysis and fluorescence in situ hybridization for ABL1 rearrangements. The patient had responded poorly to initial multi-agent chemotherapy and inotuzumab immunotherapy at relapse before the fusion was revealed. The addition of dasatinib targeting NUP214-ABL1 to inotuzumab resulted in complete molecular remission, but recurrence occurred rapidly with dasatinib alone. However, deep molecular remission was recaptured with a combination of blinatumomab and ponatinib, so he could proceed to allotransplantation. This case illustrates that next-generation sequencing approaches designed to discover cryptic gene fusions can benefit patients with Ph-like ALL.
Keywords: BCR-ABL1 l-like; NUP214-ABL1; Ph-like; acute lymphoblastic leukemia; cryptic translocation.
© The Author(s) 2022. Published by Oxford University Press.
Figures

Similar articles
-
Case Report: Specific ABL-Inhibitor Imatinib Is an Effective Targeted Agent as the First Line Therapy to Treat B-Cell Acute Lymphoblastic Leukemia With a Cryptic NUP214::ABL1 Gene Fusion.Pathol Oncol Res. 2022 Sep 12;28:1610570. doi: 10.3389/pore.2022.1610570. eCollection 2022. Pathol Oncol Res. 2022. PMID: 36172171 Free PMC article.
-
Synergistic effects of selective inhibitors targeting the PI3K/AKT/mTOR pathway or NUP214-ABL1 fusion protein in human Acute Lymphoblastic Leukemia.Oncotarget. 2016 Nov 29;7(48):79842-79853. doi: 10.18632/oncotarget.13035. Oncotarget. 2016. PMID: 27821800 Free PMC article.
-
Detection of a cryptic NUP214/ABL1 gene fusion by mate-pair sequencing (MPseq) in a newly diagnosed case of pediatric T-lymphoblastic leukemia.Cold Spring Harb Mol Case Stud. 2019 Apr 1;5(2):a003533. doi: 10.1101/mcs.a003533. Print 2019 Apr. Cold Spring Harb Mol Case Stud. 2019. PMID: 30936193 Free PMC article.
-
Rapid molecular response to dasatinib in Ph-like acute lymphoblastic leukemia patients with ABL1 rearrangements: case series and literature review.Ann Hematol. 2023 Sep;102(9):2397-2402. doi: 10.1007/s00277-023-05236-z. Epub 2023 Apr 27. Ann Hematol. 2023. PMID: 37103615 Review.
-
ABL1 fusions in T-cell acute lymphoblastic leukemia.Verh K Acad Geneeskd Belg. 2008;70(4):245-55. Verh K Acad Geneeskd Belg. 2008. PMID: 19166098 Review.
Cited by
-
Toblerone: detecting exon deletion events in cancer using RNA-seq.F1000Res. 2023 Feb 3;12:130. doi: 10.12688/f1000research.129490.1. eCollection 2023. F1000Res. 2023. PMID: 37767021 Free PMC article.
-
Clinical, Phenotypic and Molecular Characterization of NUP214-ABL1 Fusion Positive Myeloid Malignancies.J Med Cases. 2024 Sep;15(9):250-255. doi: 10.14740/jmc4286. Epub 2024 Aug 22. J Med Cases. 2024. PMID: 39205695 Free PMC article.
-
Detection of Hybrid Fusion Transcripts, Aberrant Transcript Expression, and Specific Single Nucleotide Variants in Acute Leukemia and Myeloid Disorders with Recurrent Gene Rearrangements.Pathobiology. 2024;91(1):76-88. doi: 10.1159/000532085. Epub 2023 Jul 25. Pathobiology. 2024. PMID: 37490880 Free PMC article.
References
-
- Sasaki K, Kantarjian HM, Ravandi F, et al. . Sequential combination of inotuzumab ozogamicin (ino) with low-intensity chemotherapy (mini-hyper-cvd) with or without blinatumomab is highly effective in patients (pts) with philadelphia chromosome-negative acute lymphoblastic leukemia (all) in first relapse. Blood. 2019;134(Supplement_1):3806.
-
- Tanasi I, Ba I, Sirvent N, et al. . Efficacy of tyrosine kinase inhibitors in Ph-like acute lymphoblastic leukemia harboring ABL-class rearrangements. Blood. 2019;134(16):1351-1355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

