Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products
- PMID: 35641163
- PMCID: PMC8895495
- DOI: 10.1093/stcltm/szab005
Improved MSC Minimal Criteria to Maximize Patient Safety: A Call to Embrace Tissue Factor and Hemocompatibility Assessment of MSC Products
Abstract
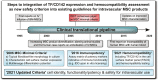
The number of mesenchymal stromal/stem cell (MSC) therapeutics and types of clinical applications have greatly diversified during the past decade, including rapid growth of poorly regulated "Stem Cell Clinics" offering diverse "Unproven Stem Cell Interventions." This product diversification necessitates a critical evaluation of the reliance on the 2006 MSC minimal criteria to not only define MSC identity but characterize MSC suitability for intravascular administration. While high-quality MSC therapeutics have been safely administered intravascularly in well-controlled clinical trials, repeated case reports of mild-to-more-severe adverse events have been reported. These are most commonly related to thromboembolic complications upon infusion of highly procoagulant tissue factor (TF/CD142)-expressing MSC products. As TF/CD142 expression varies widely depending on the source and manufacturing process of the MSC product, additional clinical cell product characterization and guidelines are needed to ensure the safe use of MSC products. To minimize risk to patients receiving MSC therapy, we here propose to supplement the minimal criteria used for characterization of MSCs, to include criteria that assess the suitability of MSC products for intravascular use. If cell products are intended for intravascular delivery, which is true for half of all clinical applications involving MSCs, the effects of MSC on coagulation and hemocompatibility should be assessed and expression of TF/CD142 should be included as a phenotypic safety marker. This adjunct criterion will ensure both the identity of the MSCs as well as the safety of the MSCs has been vetted prior to intravascular delivery of MSC products.
Keywords: cellular therapy; coagulation; coagulopathy; hemocompatibility; mesenchymal stromal/stem cells (MSCs); product diversification; safety and efficacy; thromboembolism; tissue factor/CD142/Factor III/F3; tissue source.
© The Author(s) 2022. Published by Oxford University Press.
Figures



Similar articles
-
Impact of tissue factor expression and administration routes on thrombosis development induced by mesenchymal stem/stromal cell infusions: re-evaluating the dogma.Stem Cell Res Ther. 2024 Feb 27;15(1):56. doi: 10.1186/s13287-023-03582-3. Stem Cell Res Ther. 2024. PMID: 38414067 Free PMC article.
-
MSC Therapies for COVID-19: Importance of Patient Coagulopathy, Thromboprophylaxis, Cell Product Quality and Mode of Delivery for Treatment Safety and Efficacy.Front Immunol. 2020 May 19;11:1091. doi: 10.3389/fimmu.2020.01091. eCollection 2020. Front Immunol. 2020. PMID: 32574263 Free PMC article.
-
Procoagulant activity of human mesenchymal stem cells.J Trauma Acute Care Surg. 2017 Jul;83(1 Suppl 1):S164-S169. doi: 10.1097/TA.0000000000001485. J Trauma Acute Care Surg. 2017. PMID: 28628602
-
Intravascular Mesenchymal Stromal/Stem Cell Therapy Product Diversification: Time for New Clinical Guidelines.Trends Mol Med. 2019 Feb;25(2):149-163. doi: 10.1016/j.molmed.2018.12.006. Epub 2019 Jan 30. Trends Mol Med. 2019. PMID: 30711482 Review.
-
Thrombogenic Risk Induced by Intravascular Mesenchymal Stem Cell Therapy: Current Status and Future Perspectives.Cells. 2019 Sep 27;8(10):1160. doi: 10.3390/cells8101160. Cells. 2019. PMID: 31569696 Free PMC article. Review.
Cited by
-
A STAT5-Smad3 dyad regulates adipogenic plasticity of visceral adipose mesenchymal stromal cells during chronic inflammation.NPJ Regen Med. 2022 Aug 31;7(1):41. doi: 10.1038/s41536-022-00244-5. NPJ Regen Med. 2022. PMID: 36045134 Free PMC article.
-
Low molecular weight heparin decreases pro-coagulant activity in clinical MSC products.Cytotherapy. 2024 Feb;26(2):194-200. doi: 10.1016/j.jcyt.2023.11.010. Epub 2023 Dec 21. Cytotherapy. 2024. PMID: 38127031
-
Adipose tissue-derived human mesenchymal stromal cells can better suppress complement lysis, engraft and inhibit acute graft-versus-host disease in mice.Stem Cell Res Ther. 2023 Jun 25;14(1):167. doi: 10.1186/s13287-023-03380-x. Stem Cell Res Ther. 2023. PMID: 37357314 Free PMC article.
-
Impact of tissue factor expression and administration routes on thrombosis development induced by mesenchymal stem/stromal cell infusions: re-evaluating the dogma.Stem Cell Res Ther. 2024 Feb 27;15(1):56. doi: 10.1186/s13287-023-03582-3. Stem Cell Res Ther. 2024. PMID: 38414067 Free PMC article.
-
Short-term assays for mesenchymal stromal cell immunosuppression of T-lymphocytes.Front Immunol. 2023 Sep 26;14:1225047. doi: 10.3389/fimmu.2023.1225047. eCollection 2023. Front Immunol. 2023. PMID: 37822938 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

