Human epiblast lumenogenesis: From a cell aggregate to a lumenal cyst
- PMID: 35637065
- PMCID: PMC9529837
- DOI: 10.1016/j.semcdb.2022.05.009
Human epiblast lumenogenesis: From a cell aggregate to a lumenal cyst
Abstract
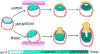
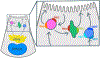
The formation of a central lumen in the human epiblast is a critical step for development. However, because the lumen forms in the epiblast coincident with implantation, the molecular and cellular events of this early lumenogenesis process cannot be studied in vivo. Recent developments using new model systems have revealed insight into the underpinnings of epiblast formation. To provide an up-to-date comprehensive review of human epiblast lumenogenesis, we highlight recent findings from human and mouse models with an emphasis on new molecular understanding of a newly described apicosome compartment, a novel 'formative' state of pluripotency that coordinates with epiblast polarization, and new evidence about the physical and polarized trafficking mechanisms contributing to lumenogenesis.
Keywords: Epiblast lumenogenesis; Epithelial cell polarity; Human epiblast; Membrane trafficking; Pluripotent stem cells.
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare no conflict of interest.
Figures

Similar articles
-
Apical polarization and lumenogenesis: The apicosome sheds new light.J Cell Biol. 2017 Dec 4;216(12):3891-3893. doi: 10.1083/jcb.201710028. Epub 2017 Nov 14. J Cell Biol. 2017. PMID: 29138252 Free PMC article.
-
Agarose microgel culture delineates lumenogenesis in naive and primed human pluripotent stem cells.Stem Cell Reports. 2021 May 11;16(5):1347-1362. doi: 10.1016/j.stemcr.2021.04.009. Stem Cell Reports. 2021. PMID: 33979603 Free PMC article.
-
An apicosome initiates self-organizing morphogenesis of human pluripotent stem cells.J Cell Biol. 2017 Dec 4;216(12):3981-3990. doi: 10.1083/jcb.201704085. Epub 2017 Oct 11. J Cell Biol. 2017. PMID: 29021220 Free PMC article.
-
Epiblast stem cells contribute new insight into pluripotency and gastrulation.Dev Growth Differ. 2010 Apr;52(3):293-301. doi: 10.1111/j.1440-169X.2010.01171.x. Epub 2010 Mar 7. Dev Growth Differ. 2010. PMID: 20298258 Review.
-
The exploration of pluripotency space: Charting cell state transitions in peri-implantation development.Cell Stem Cell. 2021 Nov 4;28(11):1896-1906. doi: 10.1016/j.stem.2021.10.001. Epub 2021 Oct 20. Cell Stem Cell. 2021. PMID: 34672948 Review.
Cited by
-
CartoCell, a high-content pipeline for 3D image analysis, unveils cell morphology patterns in epithelia.Cell Rep Methods. 2023 Oct 23;3(10):100597. doi: 10.1016/j.crmeth.2023.100597. Epub 2023 Sep 25. Cell Rep Methods. 2023. PMID: 37751739 Free PMC article.
-
Temporally resolved early BMP-driven transcriptional cascade during human amnion specification.bioRxiv [Preprint]. 2024 Mar 6:2023.06.19.545574. doi: 10.1101/2023.06.19.545574. bioRxiv. 2024. Update in: Elife. 2024 Jul 25;12:RP89367. doi: 10.7554/eLife.89367 PMID: 38496419 Free PMC article. Updated. Preprint.
-
Lumen expansion is initially driven by apical actin polymerization followed by osmotic pressure in a human epiblast model.Cell Stem Cell. 2024 May 2;31(5):640-656.e8. doi: 10.1016/j.stem.2024.03.016. Cell Stem Cell. 2024. PMID: 38701758
-
Temporally resolved early bone morphogenetic protein-driven transcriptional cascade during human amnion specification.Elife. 2024 Jul 25;12:RP89367. doi: 10.7554/eLife.89367. Elife. 2024. PMID: 39051990 Free PMC article.
-
Temporally resolved single cell transcriptomics in a human model of amniogenesis.bioRxiv [Preprint]. 2024 Aug 22:2023.09.07.556553. doi: 10.1101/2023.09.07.556553. bioRxiv. 2024. PMID: 39026707 Free PMC article. Preprint.
References
-
- Rossant J, Genetic Control of Early Cell Lineages in the Mammalian Embryo, Annu Rev Genet 52 (2018) 185–201. - PubMed
-
- Rossant J, Tam PPL, Early human embryonic development: Blastocyst formation to gastrulation, Developmental cell 57(2) (2022) 152–165. - PubMed
-
- Kinoshita M, Smith A, Pluripotency Deconstructed, Development, growth & differentiation 60(1) (2018) 44–52. - PubMed
-
- Shahbazi MN, Zernicka-Goetz M, Deconstructing and reconstructing the mouse and human early embryo, Nature cell biology (2018). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

