Clinical Diagnostic Point-of-Care Molecular Assays for SARS-CoV-2
- PMID: 35636823
- PMCID: PMC8894858
- DOI: 10.1016/j.cll.2022.03.002
Clinical Diagnostic Point-of-Care Molecular Assays for SARS-CoV-2
Abstract
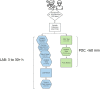
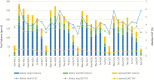
Laboratories faced many challenges throughout the COVID-19 pandemic. Point-of-care (POC) SARS-CoV-2 nucleic acid amplification tests (NAATs) provided a key solution to the need for rapid turnaround time in select patient populations and were implemented at the POC but also within laboratories to supplement traditional molecular assays. Clinical Laboratory Improvement Amendments-waived rapid POC SARS-CoV-2 NAATs offer the benefit of reduced educational requirements for operators and can be performed by non-laboratory-trained individuals. However, these methods must be validated to ensure the manufacturer's performance specifications are met and they are found to be fit-for-purpose in the clinical workflows they are implemented.
Keywords: COVID-19 pandemic; Method validation; Point-of-care testing; Rapid nucleic acid amplification tests (NAATs); SARS-CoV-2 molecular assays.
Copyright © 2022 Elsevier Inc. All rights reserved.
Figures
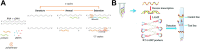


Similar articles
-
Point of care molecular and antigen detection tests for COVID-19: current status and future prospects.Expert Rev Mol Diagn. 2022 Aug;22(8):797-809. doi: 10.1080/14737159.2022.2122712. Epub 2022 Sep 14. Expert Rev Mol Diagn. 2022. PMID: 36093682 Review.
-
Advances in nucleic acid amplification techniques (NAATs): COVID-19 point-of-care diagnostics as an example.Biosens Bioelectron. 2022 Jun 15;206:114109. doi: 10.1016/j.bios.2022.114109. Epub 2022 Feb 26. Biosens Bioelectron. 2022. PMID: 35245867 Review.
-
Molecular Point-of-Care Assay Development: Design and Considerations.Curr Protoc. 2024 Jun;4(6):e1058. doi: 10.1002/cpz1.1058. Curr Protoc. 2024. PMID: 38884351
-
Detection of SARS-CoV-2 at the point of care.Bioanalysis. 2021 Aug;13(15):1213-1223. doi: 10.4155/bio-2021-0078. Epub 2021 Jul 22. Bioanalysis. 2021. PMID: 34289741 Free PMC article.
-
Comparison of an ID NOW COVID-19 Assay Used at the Point of Care to Laboratory-Based Nucleic Acid Amplification Tests.J Clin Microbiol. 2023 Jul 20;61(7):e0041323. doi: 10.1128/jcm.00413-23. Epub 2023 Jul 3. J Clin Microbiol. 2023. PMID: 37395672 Free PMC article.
Cited by
-
A novel viral RNA detection method based on the combined use of trans-acting ribozymes and HCR-FRET analyses.PLoS One. 2024 Sep 26;19(9):e0310171. doi: 10.1371/journal.pone.0310171. eCollection 2024. PLoS One. 2024. PMID: 39325749 Free PMC article.
-
Development of an integrated sample amplification control for salivary point-of-care pathogen testing.Anal Chim Acta. 2024 Jan 25;1287:342072. doi: 10.1016/j.aca.2023.342072. Epub 2023 Nov 29. Anal Chim Acta. 2024. PMID: 38182338
-
Development of an Integrated Sample Amplification Control for Salivary Point-of-Care Pathogen Testing.medRxiv [Preprint]. 2023 Oct 3:2023.10.03.23296477. doi: 10.1101/2023.10.03.23296477. medRxiv. 2023. Update in: Anal Chim Acta. 2024 Jan 25;1287:342072. doi: 10.1016/j.aca.2023.342072. PMID: 37873363 Free PMC article. Updated. Preprint.
-
Dataset from the comparative evaluation of the STANDARD M10 point-of-care analyzer versus the NeuMoDx assay for the molecular diagnosis of SARS-CoV-2.Data Brief. 2022 Dec;45:108690. doi: 10.1016/j.dib.2022.108690. Epub 2022 Oct 21. Data Brief. 2022. PMID: 36313810 Free PMC article.
References
-
- Abbott diagnostics ID NOW COVID-19 instructions for Use (Rev. 7 2020/09) https://www.fda.gov/media/136525/download Available at: Accessed July 1, 2021.
-
- Cepheid Xpert Xpress SARS-CoV-2 instructions for Use (Rev. F January 2021) https://www.fda.gov/media/136315/download Available at: Accessed July 1, 2021.
-
- Roche molecular systems cobas SARS-CoV-2 & influenza A/B nucleic acid test instructions for Use (Rev. 2.0) https://www.fda.gov/media/142193/download Available at: Accessed July 1, 2021.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

