STIM1-Orai1 Interaction Exacerbates LPS-Induced Inflammation and Endoplasmic Reticulum Stress in Bovine Hepatocytes through Store-Operated Calcium Entry
- PMID: 35627260
- PMCID: PMC9140735
- DOI: 10.3390/genes13050874
STIM1-Orai1 Interaction Exacerbates LPS-Induced Inflammation and Endoplasmic Reticulum Stress in Bovine Hepatocytes through Store-Operated Calcium Entry
Abstract
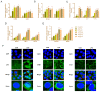
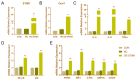
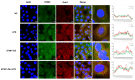
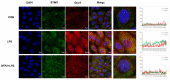
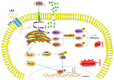
(1) Background: The basic mechanism of store-operated Ca2+ entry (SOCE) in bovine hepatocytes (BHEC) is related to the activation of STIM1 and Orai1. The effect of STIM1- and Orai1-dependent calcium ion signaling on the NF-κB signaling pathway is unclear. (2) Methods: In this study, the expression of STIM1 and Orai1 in BHEC was regulated. RT-qPCR, Western blotting, and an immunofluorescence antibody (IFA) assay were performed to elucidate the effect of inflammation and endoplasmic reticulum stress (ERS) in BHEC. (3) Results: First of all, in this study, RT-PCR and Western blotting were used to detect the levels of IκB, NF-κB, and inflammatory factors (IL-6, IL-8, and TNF-α) and the expression of genes and proteins related to ERS (PERK, IRE1, ATF6, GRP78, and CHOP), which reached peak levels simultaneously when BHEC were treated with 16 μg/mL LPS for 1 h. For STIM1, we overexpressed STIM1 in BHEC by using plasmid transfection technology. The results showed that after overexpression of STIM1, the gene and protein expression of STIM1 levels were significantly upregulated, and the expression of Orai1 on the cell membrane was also upregulated, which directly activated the SOCE channel and induced inflammation and ERS in BHEC. The overexpression group was then treated with LPS, and it was found that the overexpression of STIM1 could enhance LPS-induced BHEC inflammation and ERS in BHEC. For Orai1, BHEC were pretreated with 8 μg/mL of the specific inhibitor BTP2 for 6 h. It was found that BTP2 could inhibit the expression of mRNA in Orai1, significantly reduce the gene expression of STIM1, inhibit the activation of the NF-κB signaling pathway, and alleviate inflammation and ERS in BHEC under LPS stimulation. (4) Conclusions: In conclusion, STIM1/Orai1 can intervene and exacerbate LPS-induced inflammation and ERS in bovine hepatocytes through SOCE.
Keywords: LPS; Orai1; STIM1; endoplasmic reticulum stress; inflammation.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
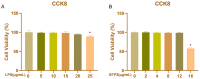


Similar articles
-
Regulation of phagocytosis and cytokine secretion by store-operated calcium entry in primary isolated murine microglia.Cell Signal. 2015 Jan;27(1):177-86. doi: 10.1016/j.cellsig.2014.11.003. Epub 2014 Nov 7. Cell Signal. 2015. PMID: 25451082
-
β-carotene alleviates LPS-induced inflammation through regulating STIM1/ORAI1 expression in bovine mammary epithelial cells.Int Immunopharmacol. 2022 Dec;113(Pt A):109377. doi: 10.1016/j.intimp.2022.109377. Epub 2022 Nov 2. Int Immunopharmacol. 2022. PMID: 36334371
-
STIM1 Regulates Endothelial Calcium Overload and Cytokine Upregulation During Sepsis.J Surg Res. 2021 Jul;263:236-244. doi: 10.1016/j.jss.2020.12.016. Epub 2021 Mar 10. J Surg Res. 2021. PMID: 33713955
-
Store-Independent Orai Channels Regulated by STIM.In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. In: Kozak JA, Putney JW Jr, editors. Calcium Entry Channels in Non-Excitable Cells. Boca Raton (FL): CRC Press/Taylor & Francis; 2018. Chapter 11. PMID: 30299650 Free Books & Documents. Review.
-
Fine-tuning of store-operated calcium entry by fast and slow Ca2+-dependent inactivation: Involvement of SARAF.Biochim Biophys Acta Mol Cell Res. 2018 Mar;1865(3):463-469. doi: 10.1016/j.bbamcr.2017.12.001. Epub 2017 Dec 6. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29223474 Review.
Cited by
-
Transcription Factor Nrf2 Modulates Lipopolysaccharide-Induced Injury in Bovine Endometrial Epithelial Cells.Int J Mol Sci. 2023 Jul 7;24(13):11221. doi: 10.3390/ijms241311221. Int J Mol Sci. 2023. PMID: 37446399 Free PMC article.
-
Genome-wide detection of copy number variation in American mink using whole-genome sequencing.BMC Genomics. 2022 Sep 13;23(1):649. doi: 10.1186/s12864-022-08874-1. BMC Genomics. 2022. PMID: 36096727 Free PMC article.
-
Targeting CaN/NFAT in Alzheimer's brain degeneration.Front Immunol. 2023 Nov 23;14:1281882. doi: 10.3389/fimmu.2023.1281882. eCollection 2023. Front Immunol. 2023. PMID: 38077352 Free PMC article. Review.
-
IGF2BP2‑dependent STIM1 inhibition protects against LPS‑induced pneumonia in vitro by alleviating endoplasmic reticulum stress and the inflammatory response.Exp Ther Med. 2023 Oct 26;26(6):575. doi: 10.3892/etm.2023.12273. eCollection 2023 Dec. Exp Ther Med. 2023. PMID: 38023363 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

