Transcriptome Analysis Reveals the Differentially Expressed Genes Associated with Growth in Guangxi Partridge Chickens
- PMID: 35627183
- PMCID: PMC9140345
- DOI: 10.3390/genes13050798
Transcriptome Analysis Reveals the Differentially Expressed Genes Associated with Growth in Guangxi Partridge Chickens
Abstract
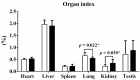
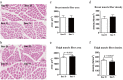
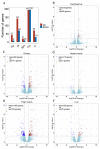
The Guangxi Partridge chicken is a well-known chicken breed in southern China with good meat quality, which has been bred as a meat breed to satisfy the increased demand of consumers. Compared with line D whose body weight is maintained at the average of the unselected group, the growth rate and weight of the selected chicken group (line S) increased significantly after breeding for four generations. Herein, transcriptome analysis was performed to identify pivotal genes and signal pathways of selective breeding that contributed to potential mechanisms of growth and development under artificial selection pressure. The average body weight of line S chickens was 1.724 kg at 90 d of age, which showed a significant increase at 90 d of age than line D chickens (1.509 kg), although only the internal organ ratios of lung and kidney changed after standardizing by body weight. The myofiber area and myofiber density of thigh muscles were affected by selection to a greater extent than that of breast muscle. We identified 51, 210, 31, 388, and 100 differentially expressed genes (DEGs) in the hypothalamus, pituitary, breast muscle, thigh muscle, and liver between the two lines, respectively. Several key genes were identified in the hypothalamus-pituitary-muscle axis, such as FST, THSB, PTPRJ, CD36, PITX1, PITX2, AMPD1, PRKAB1, PRKAB2, and related genes for muscle development, which were attached to the cytokine-cytokine receptor interaction signaling pathway, the PPAR signaling pathway, and lipid metabolism. However, signaling molecular pathways and the cell community showed that elevated activity in the liver of line S fowl was mainly involved in focal adhesion, ECM-receptor interaction, cell adhesion molecules, and signal transduction. Collectively, muscle development, lipid metabolism, and several signaling pathways played crucial roles in the improving growth performance of Guangxi Partridge chickens under artificial selection for growth rate. These results support further study of the adaptation of birds under selective pressure.
Keywords: Guangxi Partridge chicken; breeding; growth rate; hypothalamus; liver; muscle; pituitary; transcriptome.
Conflict of interest statement
Y.T. is an employee of Guangxi Fufeng Agricultural and Animal Husbandry Group Co., Ltd. that did not provide financial support to this study. All the other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



Similar articles
-
Transcriptional insights into key genes and pathways controlling muscle lipid metabolism in broiler chickens.BMC Genomics. 2019 Nov 15;20(1):863. doi: 10.1186/s12864-019-6221-0. BMC Genomics. 2019. PMID: 31729950 Free PMC article.
-
Identification of differentially expressed genes and pathways for intramuscular fat deposition in pectoralis major tissues of fast-and slow-growing chickens.BMC Genomics. 2012 May 30;13:213. doi: 10.1186/1471-2164-13-213. BMC Genomics. 2012. PMID: 22646994 Free PMC article.
-
Identification of Differentially Expressed Genes and Pathways for Myofiber Characteristics in Soleus Muscles between Chicken Breeds Differing in Meat Quality.Anim Biotechnol. 2017 Apr 3;28(2):83-93. doi: 10.1080/10495398.2016.1206555. Epub 2016 Sep 13. Anim Biotechnol. 2017. PMID: 27623936
-
Transcriptomic analysis reveals differentially expressed genes associated with meat quality in Chinese Dagu chicken and AA+ broiler roosters.BMC Genomics. 2024 Oct 26;25(1):1002. doi: 10.1186/s12864-024-10927-6. BMC Genomics. 2024. PMID: 39455924 Free PMC article.
-
RNA sequencing for global gene expression associated with muscle growth in a single male modern broiler line compared to a foundational Barred Plymouth Rock chicken line.BMC Genomics. 2017 Jan 13;18(1):82. doi: 10.1186/s12864-016-3471-y. BMC Genomics. 2017. PMID: 28086790 Free PMC article.
Cited by
-
Multi-omics analysis reveals associations between host gene expression, gut microbiota, and metabolites in chickens.J Anim Sci. 2024 Jan 3;102:skae263. doi: 10.1093/jas/skae263. J Anim Sci. 2024. PMID: 39243135
-
Genome-wide association analysis identify candidate genes for feed efficiency and growth traits in Wenchang chickens.BMC Genomics. 2024 Jun 28;25(1):645. doi: 10.1186/s12864-024-10559-w. BMC Genomics. 2024. PMID: 38943081 Free PMC article.
-
Transcriptome analysis reveals the pathogenesis of spontaneous tibial dyschondroplasia in broilers.Front Genet. 2024 Jul 29;15:1434532. doi: 10.3389/fgene.2024.1434532. eCollection 2024. Front Genet. 2024. PMID: 39139824 Free PMC article.
-
Dynamic Changes in Intestinal Gene Expression and Microbiota across Chicken Egg-Laying Stages.Animals (Basel). 2024 May 22;14(11):1529. doi: 10.3390/ani14111529. Animals (Basel). 2024. PMID: 38891577 Free PMC article.
-
Untargeted Metabolomics Reveals the Effect of Selective Breeding on the Quality of Chicken Meat.Metabolites. 2022 Apr 19;12(5):367. doi: 10.3390/metabo12050367. Metabolites. 2022. PMID: 35629871 Free PMC article.
References
-
- Maharjan P., Martinez D.A., Weil J., Suesuttajit N., Umberson C., Mullenix G., Hilton K.M., Beitia A., Coon C.N. Review: Physiological growth trend of current meat broilers and dietary protein and energy management approaches for sustainable broiler production. Anim. Int. J. Anim. Biosci. 2021;15:100284. doi: 10.1016/j.animal.2021.100284. - DOI - PubMed
-
- Zhang Z., Du H., Yang C., Li Q., Qiu M., Song X., Yu C., Jiang X., Liu L., Hu C., et al. Comparative transcriptome analysis reveals regulators mediating breast muscle growth and development in three chicken breeds. Anim. Biotechnol. 2019;30:233–241. doi: 10.1080/10495398.2018.1476377. - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

