Interleukin-6 Signaling in Triple Negative Breast Cancer Cells Elicits the Annexin A1/Formyl Peptide Receptor 1 Axis and Affects the Tumor Microenvironment
- PMID: 35626741
- PMCID: PMC9139391
- DOI: 10.3390/cells11101705
Interleukin-6 Signaling in Triple Negative Breast Cancer Cells Elicits the Annexin A1/Formyl Peptide Receptor 1 Axis and Affects the Tumor Microenvironment
Abstract
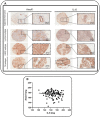
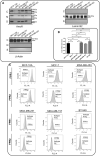
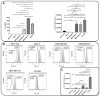
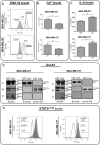
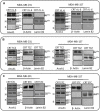
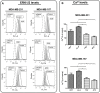
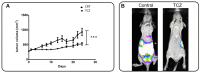
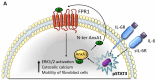
Annexin A1 (AnxA1) is a pleiotropic protein that exerts essential roles in breast cancer (BC) growth and aggressiveness. In our previous work, we described the autocrine signaling of AnxA1 through formyl peptide receptor 1 (FPR1) in the triple-negative (TN) BC cell line, MDA-MB-231. Here, we aimed to describe the interaction between the AnxA1/FPR1 and the Interleukin-6 (IL-6) signaling pathways and their role in the tumor microenvironment (TME). First, we demonstrated that AnxA1 and IL-6 expression levels are correlated in BC tissue samples. In three TNBC cell lines, overexpression of both AnxA1 and IL-6 was also identified. Next, we inhibited FPR1, the IL-6 receptor and STAT3 in both MDA-MB-231 and MDA-MB-157 cells. The FPR1 inhibition led to increased levels of IL-6 and secreted AnxA1 in both cell lines. On the other side, inhibition of the IL-6 receptor or STAT3 led to the impairment of AnxA1 secretion, suggesting the essential role of the IL-6 signaling cascade in the activation of the AnxA1/FPR1 autocrine axis. Finally, we described the interaction between IL-6 and the AnxA1/FPR1 pathways and their role on the TME by analyzing the effect of supernatants derived from MDA-MB-231 and MDA-MB-157 cells under the inhibition of FPR1 or IL-6 signaling on fibroblast cell motility.
Keywords: Annexin A1; IL-6; STAT3; autocrine signaling; breast cancer; formyl peptide receptor.
Conflict of interest statement
The authors report no declarations of interest.
Figures



Similar articles
-
An overview of the regulatory role of annexin A1 in the tumor microenvironment and its prospective clinical application (Review).Int J Oncol. 2024 May;64(5):51. doi: 10.3892/ijo.2024.5639. Epub 2024 Mar 22. Int J Oncol. 2024. PMID: 38516766 Free PMC article. Review.
-
Inhibition of the AnxA1/FPR1 autocrine axis reduces MDA-MB-231 breast cancer cell growth and aggressiveness in vitro and in vivo.Biochim Biophys Acta Mol Cell Res. 2018 Sep;1865(9):1368-1382. doi: 10.1016/j.bbamcr.2018.06.010. Epub 2018 Jun 20. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 29932988
-
Breast cancer metastasis to brain results in recruitment and activation of microglia through annexin-A1/formyl peptide receptor signaling.Breast Cancer Res. 2022 Apr 5;24(1):25. doi: 10.1186/s13058-022-01514-2. Breast Cancer Res. 2022. PMID: 35382852 Free PMC article.
-
Annexin A1/Formyl Peptide Receptor Pathway Controls Uterine Receptivity to the Blastocyst.Cells. 2020 May 11;9(5):1188. doi: 10.3390/cells9051188. Cells. 2020. PMID: 32403233 Free PMC article.
-
Annexin A1 localization and its relevance to cancer.Clin Sci (Lond). 2016 Feb;130(4):205-20. doi: 10.1042/CS20150415. Clin Sci (Lond). 2016. PMID: 26769657 Review.
Cited by
-
Hypoxia: syndicating triple negative breast cancer against various therapeutic regimens.Front Oncol. 2023 Jul 10;13:1199105. doi: 10.3389/fonc.2023.1199105. eCollection 2023. Front Oncol. 2023. PMID: 37492478 Free PMC article. Review.
-
Expression profile of interleukin-6, 4-hydroxy-2-nonenal, and hypoxia-inducible factor 1-α in women with breast cancer and their association with clinicopathological parameters.Contemp Oncol (Pozn). 2023;27(1):14-21. doi: 10.5114/wo.2023.127199. Epub 2023 Apr 27. Contemp Oncol (Pozn). 2023. PMID: 37266334 Free PMC article.
-
Co-Encapsulation of Paclitaxel and JQ1 in Zein Nanoparticles as Potential Innovative Nanomedicine.Micromachines (Basel). 2022 Sep 22;13(10):1580. doi: 10.3390/mi13101580. Micromachines (Basel). 2022. PMID: 36295933 Free PMC article.
-
An overview of the regulatory role of annexin A1 in the tumor microenvironment and its prospective clinical application (Review).Int J Oncol. 2024 May;64(5):51. doi: 10.3892/ijo.2024.5639. Epub 2024 Mar 22. Int J Oncol. 2024. PMID: 38516766 Free PMC article. Review.
-
Unveiling Atherosclerotic Plaque Heterogeneity and SPP1+/VCAN+ Macrophage Subtype Prognostic Significance Through Integrative Single-Cell and Bulk-Seq Analysis.J Inflamm Res. 2024 Apr 23;17:2399-2426. doi: 10.2147/JIR.S454505. eCollection 2024. J Inflamm Res. 2024. PMID: 38681071 Free PMC article.
References
-
- Rossing M., Pedersen C.B., Tvedskov T., Vejborg I., Talman M.-L., Olsen L.R., Kroman N., Nielsen F.C., Jensen M.-B., Ejlertsen B. Clinical implications of intrinsic molecular subtypes of breast cancer for sentinel node status. Sci. Rep. 2021;11:2259. doi: 10.1038/s41598-021-81538-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

