Phase II Trial of CDX-3379 and Cetuximab in Recurrent/Metastatic, HPV-Negative, Cetuximab-Resistant Head and Neck Cancer
- PMID: 35625959
- PMCID: PMC9139981
- DOI: 10.3390/cancers14102355
Phase II Trial of CDX-3379 and Cetuximab in Recurrent/Metastatic, HPV-Negative, Cetuximab-Resistant Head and Neck Cancer
Abstract
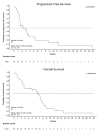
In phase I development, CDX-3379, an anti-ErbB3 monoclonal antibody, showed promising molecular and antitumor activity in head and neck squamous cell carcinoma (HNSCC), alone or in combination with cetuximab. Preliminary biomarker data raised the hypothesis of enhanced response in tumors harboring FAT1 mutations. This phase II, multicenter trial used a Simon 2-stage design to investigate the efficacy of CDX-3379 and cetuximab in 30 patients with recurrent/metastatic, HPV-negative, cetuximab-resistant HNSCC. The primary endpoint was objective response rate (ORR). Secondary endpoints included ORR in patients with somatic FAT1 mutations, progression-free survival (PFS), overall survival (OS), and safety. Thirty patients were enrolled from March 2018 to September 2020. The ORR in genomically unselected patients was 2/30 (6.7%; 95% confidence interval [CI], 0.8-22.1). Median PFS and OS were 2.2 (95% CI: 1.3-3.6) and 6.6 months (95% CI: 2.7-7.5), respectively. Tissue was available in 27 patients including one of two responders. ORR was 1/10 (complete response; 10%; 95% CI 0.30-44.5) in the FAT1-mutated versus 0/17 (0%; 95% CI: 0-19.5) in the FAT1-wildtype cohorts. Sixteen patients (53%) experienced treatment-related adverse events (AEs) ≥ grade 3. The most common AEs were diarrhea (83%) and acneiform dermatitis (53%). Dose modification was required in 21 patients (70%). The modest ORR coupled with excessive, dose-limiting toxicity of this combination precludes further clinical development. Dual ErbB3-EGFR inhibition remains of scientific interest in HPV-negative HNSCC. Should more tolerable combinations be identified, development in an earlier line of therapy and prospective evaluation of the FAT1 hypothesis warrant consideration.
Keywords: CDX-3379; EGFR; ErbB3; cetuximab; head and neck cancer.
Conflict of interest statement
M.H.-C., D.A., R.G. and P.G. are employed by Celldex Therapeutics. The remaining authors declare no potential conflict of interest.
Figures
Similar articles
-
Randomized Phase II Trial of Ficlatuzumab With or Without Cetuximab in Pan-Refractory, Recurrent/Metastatic Head and Neck Cancer.J Clin Oncol. 2023 Aug 1;41(22):3851-3862. doi: 10.1200/JCO.22.01994. Epub 2023 Mar 28. J Clin Oncol. 2023. PMID: 36977289 Clinical Trial.
-
A randomized, phase II trial of cetuximab with or without PX-866, an irreversible oral phosphatidylinositol 3-kinase inhibitor, in patients with relapsed or metastatic head and neck squamous cell cancer.Ann Oncol. 2015 Mar;26(3):556-61. doi: 10.1093/annonc/mdu574. Epub 2014 Dec 18. Ann Oncol. 2015. PMID: 25524478 Clinical Trial.
-
Molecular and Clinical Activity of CDX-3379, an Anti-ErbB3 Monoclonal Antibody, in Head and Neck Squamous Cell Carcinoma Patients.Clin Cancer Res. 2019 Oct 1;25(19):5752-5758. doi: 10.1158/1078-0432.CCR-18-3453. Epub 2019 Jul 15. Clin Cancer Res. 2019. PMID: 31308059 Free PMC article. Clinical Trial.
-
[Cetuximab in head and neck squamous cell carcinoma: a systematic review and Meta-analysis].Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015 Jan;29(1):67-75. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015. PMID: 25966559 Review. Chinese.
-
Cetuximab-Containing Combinations in Locally Advanced and Recurrent or Metastatic Head and Neck Squamous Cell Carcinoma.Front Oncol. 2019 May 20;9:383. doi: 10.3389/fonc.2019.00383. eCollection 2019. Front Oncol. 2019. PMID: 31165040 Free PMC article. Review.
Cited by
-
Human Papilloma Virus Positive Oropharyngeal Squamous Cell Carcinoma and the Immune System: Pathogenesis, Immunotherapy and Future Perspectives.Int J Mol Sci. 2024 Feb 28;25(5):2798. doi: 10.3390/ijms25052798. Int J Mol Sci. 2024. PMID: 38474047 Free PMC article. Review.
-
HER3 Alterations in Cancer and Potential Clinical Implications.Cancers (Basel). 2022 Dec 14;14(24):6174. doi: 10.3390/cancers14246174. Cancers (Basel). 2022. PMID: 36551663 Free PMC article. Review.
-
Tislelizumab plus nimotuzumab is effective against recurrent or metastatic oral squamous cell carcinoma among patients with a performance status score ≥ 2: a retrospective study.Front Oncol. 2024 Jan 16;13:1273798. doi: 10.3389/fonc.2023.1273798. eCollection 2023. Front Oncol. 2024. PMID: 38293699 Free PMC article.
-
The diverse functions of FAT1 in cancer progression: good, bad, or ugly?J Exp Clin Cancer Res. 2022 Aug 15;41(1):248. doi: 10.1186/s13046-022-02461-8. J Exp Clin Cancer Res. 2022. PMID: 35965328 Free PMC article. Review.
References
-
- Ang K.K., Berkey B.A., Tu X., Zhang H.Z., Katz R., Hammond E.H., Fu K.K., Milas L. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous