Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy
- PMID: 35625674
- PMCID: PMC9138693
- DOI: 10.3390/biomedicines10050937
Experimental Models for Testing the Efficacy of Pharmacological Treatments for Neonatal Hypoxic-Ischemic Encephalopathy
Abstract
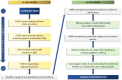
Representing an important cause of long-term disability, term neonatal hypoxic-ischemic encephalopathy (HIE) urgently needs further research aimed at repurposing existing drug as well as developing new therapeutics. Since various experimental in vitro and in vivo models of HIE have been developed with distinct characteristics, it becomes important to select the appropriate preclinical screening cascade for testing the efficacy of novel pharmacological treatments. As therapeutic hypothermia is already a routine therapy for neonatal encephalopathy, it is essential that hypothermia be administered to the experimental model selected to allow translational testing of novel or repurposed drugs on top of the standard of care. Moreover, a translational approach requires that therapeutic interventions must be initiated after the induction of the insult, and the time window for intervention should be evaluated to translate to real world clinical practice. Hippocampal organotypic slice cultures, in particular, are an invaluable intermediate between simpler cell lines and in vivo models, as they largely maintain structural complexity of the original tissue and can be subjected to transient oxygen-glucose deprivation (OGD) and subsequent reoxygenation to simulate ischemic neuronal injury and reperfusion. Progressing to in vivo models, generally, rodent (mouse and rat) models could offer more flexibility and be more cost-effective for testing the efficacy of pharmacological agents with a dose-response approach. Large animal models, including piglets, sheep, and non-human primates, may be utilized as a third step for more focused and accurate translational studies, including also pharmacokinetic and safety pharmacology assessments. Thus, a preclinical proof of concept of efficacy of an emerging pharmacological treatment should be obtained firstly in vitro, including organotypic models, and, subsequently, in at least two different animal models, also in combination with hypothermia, before initiating clinical trials.
Keywords: cell cultures; cerebral ischemia; drug development; efficacy studies; hypoxia; neonate animal models; organotypic hippocampal slices; oxygen and glucose deprivation; toxicological studies.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Neuroprotective effects of topiramate and memantine in combination with hypothermia in hypoxic-ischemic brain injury in vitro and in vivo.Neurosci Lett. 2018 Mar 6;668:103-107. doi: 10.1016/j.neulet.2018.01.023. Epub 2018 Jan 12. Neurosci Lett. 2018. PMID: 29339173
-
Endogenous hypothermic response to hypoxia reduces brain injury: Implications for modeling hypoxic-ischemic encephalopathy and therapeutic hypothermia in neonatal mice.Exp Neurol. 2016 Sep;283(Pt A):264-75. doi: 10.1016/j.expneurol.2016.06.024. Epub 2016 Jun 25. Exp Neurol. 2016. PMID: 27349408
-
Melatonin Acts in Synergy with Hypothermia to Reduce Oxygen-Glucose Deprivation-Induced Cell Death in Rat Hippocampus Organotypic Slice Cultures.Neonatology. 2018;114(4):364-371. doi: 10.1159/000491859. Epub 2018 Aug 28. Neonatology. 2018. PMID: 30153673
-
Stem cell therapy for neonatal hypoxic-ischemic encephalopathy.Front Neurol. 2014 Aug 12;5:147. doi: 10.3389/fneur.2014.00147. eCollection 2014. Front Neurol. 2014. PMID: 25161645 Free PMC article. Review.
-
Hydrogen-induced Neuroprotection in Neonatal Hypoxic-ischemic Encephalopathy.Curr Pharm Des. 2021;27(5):687-694. doi: 10.2174/1381612826666201113095720. Curr Pharm Des. 2021. PMID: 33185158 Review.
Cited by
-
A Novel Non-Invasive Murine Model of Neonatal Hypoxic-Ischemic Encephalopathy Demonstrates Developmental Delay and Motor Deficits with Activation of Inflammatory Pathways in Monocytes.Cells. 2024 Sep 14;13(18):1551. doi: 10.3390/cells13181551. Cells. 2024. PMID: 39329733 Free PMC article.
-
Research progress of neonatal hypoxic-ischemic encephalopathy in nonhuman primate models.Ibrain. 2023 Mar 26;9(2):183-194. doi: 10.1002/ibra.12097. eCollection 2023 Summer. Ibrain. 2023. PMID: 37786551 Free PMC article. Review.
-
Current analysis of hypoxic-ischemic encephalopathy research issues and future treatment modalities.Front Neurosci. 2023 Jun 9;17:1136500. doi: 10.3389/fnins.2023.1136500. eCollection 2023. Front Neurosci. 2023. PMID: 37360183 Free PMC article. Review.
-
Dried Blood Spot Metabolome Features of Ischemic-Hypoxic Encephalopathy: A Neonatal Rat Model.Int J Mol Sci. 2024 Aug 15;25(16):8903. doi: 10.3390/ijms25168903. Int J Mol Sci. 2024. PMID: 39201589 Free PMC article.
-
Experimental Modeling of Damaging and Protective Hypoxia of the Mammalian Brain.J Evol Biochem Physiol. 2022;58(6):2021-2034. doi: 10.1134/S0022093022060291. Epub 2022 Dec 22. J Evol Biochem Physiol. 2022. PMID: 36573160 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

