Study on the Neuroprotective, Radical-Scavenging and MAO-B Inhibiting Properties of New Benzimidazole Arylhydrazones as Potential Multi-Target Drugs for the Treatment of Parkinson's Disease
- PMID: 35624746
- PMCID: PMC9138090
- DOI: 10.3390/antiox11050884
Study on the Neuroprotective, Radical-Scavenging and MAO-B Inhibiting Properties of New Benzimidazole Arylhydrazones as Potential Multi-Target Drugs for the Treatment of Parkinson's Disease
Abstract
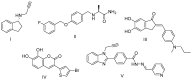
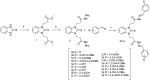
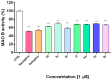
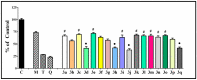
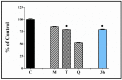
Oxidative stress is a key contributing factor in the complex degenerating cascade in Parkinson's disease. The inhibition of MAO-B affords higher dopamine bioavailability and stops ROS formation. The incorporation of hydroxy and methoxy groups in the arylhydrazone moiety of a new series of 1,3-disubstituted benzimidazole-2-thiones could increase the neuroprotective activity. In vitro safety evaluation on SH-SY5Y cells and rat brain synaptosomes showed a strong safety profile. Antioxidant and neuroprotective effects were evaluated in H2O2-induced oxidative stress on SH-SY5Y cells and in a model of 6-OHDA-induced neurotoxicity in rat brain synaptosomes, where the dihydroxy compounds 3h and 3i demonstrated the most robust neuroprotective and antioxidant activity, more pronounced than the reference melatonin and rasagiline. Statistically significant MAO-B inhibitory effects were exerted by some of the compounds where again the catecholic compound 3h was the most potent inhibitor similar to selegiline and rasagiline. The most potent antioxidant effect in the ferrous iron induced lipid peroxidation assay was observed for the three catechols-3h and 3j, 3q. The catecholic compound 3h showed scavenging capability against superoxide radicals and antioxidant effect in the iron/deoxyribose system. The study outlines a perspective multifunctional compound with the best safety profile, neuroprotective, antioxidant and MAO-B inhibiting properties.
Keywords: MAO-B inhibition; MTDLs; Parkinson’s disease; benzimidazoles; catechols; lipid peroxidation; neuroprotection; superoxide scavenging; synaptosomes; synthesis.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
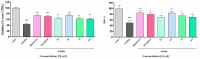




Similar articles
-
New Indole-3-Propionic Acid and 5-Methoxy-Indole Carboxylic Acid Derived Hydrazone Hybrids as Multifunctional Neuroprotectors.Antioxidants (Basel). 2023 Apr 21;12(4):977. doi: 10.3390/antiox12040977. Antioxidants (Basel). 2023. PMID: 37107353 Free PMC article.
-
Evaluation of the combined activity of benzimidazole arylhydrazones as new anti-Parkinsonian agents: monoamine oxidase-B inhibition, neuroprotection and oxidative stress modulation.Neural Regen Res. 2021 Nov;16(11):2299-2309. doi: 10.4103/1673-5374.309843. Neural Regen Res. 2021. PMID: 33818516 Free PMC article.
-
Radical Scavenging Mechanisms of 1-Arylhydrazone Benzimidazole Hybrids with Neuroprotective Activity.J Phys Chem B. 2023 May 25;127(20):4364-4373. doi: 10.1021/acs.jpcb.2c05784. Epub 2023 May 10. J Phys Chem B. 2023. PMID: 37163390
-
Pharmacological aspects of the neuroprotective effects of irreversible MAO-B inhibitors, selegiline and rasagiline, in Parkinson's disease.J Neural Transm (Vienna). 2018 Nov;125(11):1735-1749. doi: 10.1007/s00702-018-1853-9. Epub 2018 Feb 7. J Neural Transm (Vienna). 2018. PMID: 29417334 Review.
-
Bifunctional drug derivatives of MAO-B inhibitor rasagiline and iron chelator VK-28 as a more effective approach to treatment of brain ageing and ageing neurodegenerative diseases.Mech Ageing Dev. 2005 Feb;126(2):317-26. doi: 10.1016/j.mad.2004.08.023. Mech Ageing Dev. 2005. PMID: 15621213 Review.
Cited by
-
Discovery of novel 2-(4-(benzyloxy)-5-(hydroxyl) phenyl) benzothiazole derivatives as multifunctional MAO-B inhibitors for the treatment of Parkinson's disease.J Enzyme Inhib Med Chem. 2023 Dec;38(1):2159957. doi: 10.1080/14756366.2022.2159957. J Enzyme Inhib Med Chem. 2023. PMID: 36728713 Free PMC article.
-
New Indole-3-Propionic Acid and 5-Methoxy-Indole Carboxylic Acid Derived Hydrazone Hybrids as Multifunctional Neuroprotectors.Antioxidants (Basel). 2023 Apr 21;12(4):977. doi: 10.3390/antiox12040977. Antioxidants (Basel). 2023. PMID: 37107353 Free PMC article.
-
Editorial: Experimental and innovative approaches to multi-target treatment of Parkinson's and Alzheimer's diseases - Volume II.Front Neurosci. 2023 Mar 24;17:1171866. doi: 10.3389/fnins.2023.1171866. eCollection 2023. Front Neurosci. 2023. PMID: 37034172 Free PMC article. No abstract available.
-
Cycloartane Saponins from Astragalus glycyphyllos and Their In Vitro Neuroprotective, Antioxidant, and hMAO-B-Inhibiting Effects.Metabolites. 2023 Jul 19;13(7):857. doi: 10.3390/metabo13070857. Metabolites. 2023. PMID: 37512564 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

