A Chemical Mutagenesis Approach to Insert Post-translational Modifications in Aggregation-Prone Proteins
- PMID: 35609278
- PMCID: PMC9204764
- DOI: 10.1021/acschemneuro.2c00077
A Chemical Mutagenesis Approach to Insert Post-translational Modifications in Aggregation-Prone Proteins
Abstract
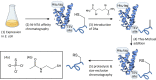
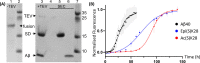
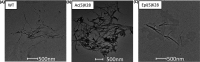
Neurodegenerative diseases are a class of disorders linked to the formation in the nervous system of fibrillar protein aggregates called amyloids. This aggregation process is affected by a variety of post-translational modifications, whose specific mechanisms are not fully understood yet. Emerging chemical mutagenesis technology is currently striving to address the challenge of introducing protein post-translational modifications, while maintaining the stability and solubility of the proteins during the modification reaction. Several amyloidogenic proteins are highly aggregation-prone, and current modification procedures can lead to unexpected precipitation of these proteins, affecting their yield and downstream characterization. Here, we present a method for maintaining amyloidogenic protein solubility during chemical mutagenesis. As proof-of-principle, we applied our method to mimic the phosphorylation of serine-26 and the acetylation of lysine-28 of the 40-residue long variant of amyloid-β peptide, whose aggregation is linked to Alzheimer's disease.
Keywords: Alzheimer’s disease; amyloid-β; chemical mutagenesis; post-translational modification.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
The inhibitory effect and mechanism of small molecules on acetic anhydride-induced BSA acetylation and aggregation.Colloids Surf B Biointerfaces. 2023 May;225:113265. doi: 10.1016/j.colsurfb.2023.113265. Epub 2023 Mar 15. Colloids Surf B Biointerfaces. 2023. PMID: 36931043
-
Gallic acid oxidation products alter the formation pathway of insulin amyloid fibrils.Sci Rep. 2020 Sep 2;10(1):14466. doi: 10.1038/s41598-020-70982-3. Sci Rep. 2020. PMID: 32879381 Free PMC article.
-
Peroxynitric acid inhibits amyloid β aggregation.Biochem Biophys Res Commun. 2023 Jun 11;660:1-5. doi: 10.1016/j.bbrc.2023.03.060. Epub 2023 Apr 6. Biochem Biophys Res Commun. 2023. PMID: 37058842
-
Deciphering an interplay of proteins associated with amyloid β 1-42 peptide and molecular mechanisms of Alzheimer's disease.Rev Neurosci. 2014;25(6):773-83. doi: 10.1515/revneuro-2014-0025. Rev Neurosci. 2014. PMID: 25010778 Review.
-
Simulation Studies of Amyloidogenic Polypeptides and Their Aggregates.Chem Rev. 2019 Jun 26;119(12):6956-6993. doi: 10.1021/acs.chemrev.8b00731. Epub 2019 Apr 11. Chem Rev. 2019. PMID: 30973229 Review.
Cited by
-
Phase separation and pathologic transitions of RNP condensates in neurons: implications for amyotrophic lateral sclerosis, frontotemporal dementia and other neurodegenerative disorders.Front Mol Neurosci. 2023 Sep 1;16:1242925. doi: 10.3389/fnmol.2023.1242925. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37720552 Free PMC article. Review.
-
Kinetic Resolution of Epimeric Proteins Enables Stereoselective Chemical Mutagenesis.J Am Chem Soc. 2024 Aug 14;146(32):22622-22628. doi: 10.1021/jacs.4c07103. Epub 2024 Jul 31. J Am Chem Soc. 2024. PMID: 39083370 Free PMC article.
References
-
- Alzheimer’s Disease International . World Alzheimer Report 2021: Journey through the Diagnosis of Dementia; 2021.
-
- Nilsberth C.; Westlind-Danielsson A.; Eckman C. B.; Condron M. M.; Axelman K.; Forsell C.; Stenh C.; Luthman J.; Teplow D. B.; Younkin S. G.; Näslund J.; Lannfelt L. The “Arctic” APP Mutation (E693G) Causes Alzheimer’s Disease by Enhanced Aβ Protofibril Formation. Nat. Neurosci. 2001, 4 (9), 887–893. 10.1038/nn0901-887. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

