Temporin B Forms Hetero-Oligomers with Temporin L, Modifies Its Membrane Activity, and Increases the Cooperativity of Its Antibacterial Pharmacodynamic Profile
- PMID: 35609188
- PMCID: PMC9178791
- DOI: 10.1021/acs.biochem.1c00762
Temporin B Forms Hetero-Oligomers with Temporin L, Modifies Its Membrane Activity, and Increases the Cooperativity of Its Antibacterial Pharmacodynamic Profile
Abstract
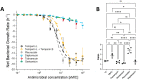
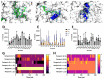
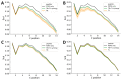
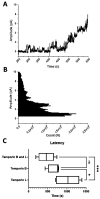
The pharmacodynamic profile of antimicrobial peptides (AMPs) and their in vivo synergy are two factors that are thought to restrict resistance evolution and ensure their conservation. The frog Rana temporaria secretes a family of closely related AMPs, temporins A-L, as an effective chemical dermal defense. The antibacterial potency of temporin L has been shown to increase synergistically in combination with both temporins B and A, but this is modest. Here we show that the less potent temporin B enhances the cooperativity of the in vitro antibacterial activity of the more potent temporin L against EMRSA-15 and that this may be associated with an altered interaction with the bacterial plasma membrane, a feature critical for the antibacterial activity of most AMPs. Addition of buforin II, a histone H2A fragment, can further increase the cooperativity. Molecular dynamics simulations indicate temporins B and L readily form hetero-oligomers in models of Gram-positive bacterial plasma membranes. Patch-clamp studies show transmembrane ion conductance is triggered with lower amounts of both peptides and more quickly when used in combination, but conductance is of a lower amplitude and pores are smaller. Temporin B may therefore act by forming temporin L/B hetero-oligomers that are more effective than temporin L homo-oligomers at bacterial killing and/or by reducing the probability of the latter forming until a threshold concentration is reached. Exploration of the mechanism of synergy between AMPs isolated from the same organism may therefore yield antibiotic combinations with advantageous pharmacodynamic properties.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
Similar articles
-
Temporin L and aurein 2.5 have identical conformations but subtly distinct membrane and antibacterial activities.Sci Rep. 2019 Jul 29;9(1):10934. doi: 10.1038/s41598-019-47327-w. Sci Rep. 2019. PMID: 31358802 Free PMC article.
-
Minor sequence modifications in temporin B cause drastic changes in antibacterial potency and selectivity by fundamentally altering membrane activity.Sci Rep. 2019 Feb 4;9(1):1385. doi: 10.1038/s41598-018-37630-3. Sci Rep. 2019. PMID: 30718667 Free PMC article.
-
Temporins: An Approach of Potential Pharmaceutic Candidates.Surg Infect (Larchmt). 2020 May;21(4):309-322. doi: 10.1089/sur.2019.266. Epub 2019 Dec 4. Surg Infect (Larchmt). 2020. PMID: 31804896 Review.
-
Interactions of the antimicrobial peptides temporins with model biomembranes. Comparison of temporins B and L.Biochemistry. 2002 Apr 2;41(13):4425-36. doi: 10.1021/bi011929e. Biochemistry. 2002. PMID: 11914090
-
An intimate link between antimicrobial peptide sequence diversity and binding to essential components of bacterial membranes.Biochim Biophys Acta. 2016 May;1858(5):958-70. doi: 10.1016/j.bbamem.2015.10.011. Epub 2015 Oct 21. Biochim Biophys Acta. 2016. PMID: 26498397 Review.
Cited by
-
Emergent conformational and aggregation properties of synergistic antimicrobial peptide combinations.Nanoscale. 2024 Nov 13;16(44):20657-20669. doi: 10.1039/d4nr03043e. Nanoscale. 2024. PMID: 39422704 Free PMC article.
-
Vesicle protrusion induced by antimicrobial peptides suggests common carpet mechanism for short antimicrobial peptides.Sci Rep. 2024 Apr 27;14(1):9701. doi: 10.1038/s41598-024-60601-w. Sci Rep. 2024. PMID: 38678109 Free PMC article.
-
QSAR Reveals Decreased Lipophilicity of Polar Residues Determines the Selectivity of Antimicrobial Peptide Activity.ACS Omega. 2024 Jun 3;9(24):26030-26049. doi: 10.1021/acsomega.4c01277. eCollection 2024 Jun 18. ACS Omega. 2024. PMID: 38911757 Free PMC article.
-
Synergistic Combination of Antimicrobial Peptides and Cationic Polyitaconates in Multifunctional PLA Fibers.ACS Appl Bio Mater. 2023 Nov 20;6(11):4805-4813. doi: 10.1021/acsabm.3c00576. Epub 2023 Oct 20. ACS Appl Bio Mater. 2023. PMID: 37862451 Free PMC article.
-
Synergy between Winter Flounder antimicrobial peptides.NPJ Antimicrob Resist. 2023;1(1):8. doi: 10.1038/s44259-023-00010-7. Epub 2023 Aug 10. NPJ Antimicrob Resist. 2023. PMID: 38686212 Free PMC article.
References
-
- Spohn R.; Daruka L.; Lázár V.; Martins A.; Vidovics F.; Grézal G.; Méhi O.; Kintses B.; Számel M.; Jangir P. K.; Csörgő B.; Györkei A.; Bódi Z.; Faragó A.; Bodai L.; Földesi I.; Kata D.; Maróti G.; Pap B.; Wirth R.; Papp B.; Pál C. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 2019, 10, 4538.10.1038/s41467-019-12364-6. - DOI - PMC - PubMed
-
- Yang Q.; Li M.; Spiller O. B.; Andrey D. O.; Hinchliffe P.; Li H.; MacLean C.; Niumsup P.; Powell L.; Pritchard M.; Papkou A.; Shen Y.; Portal E.; Sands K.; Spencer J.; Tansawai U.; Thomas D.; Wang S.; Wang Y.; Shen J.; Walsh T. Balancing mcr-1 expression and bacterial survival is a delicate equilibrium between essential cellular defence mechanisms. Nat. Commun. 2017, 8, 2054.10.1038/s41467-017-02149-0. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

