DLK-Dependent Biphasic Reactivation of Herpes Simplex Virus Latency Established in the Absence of Antivirals
- PMID: 35608347
- PMCID: PMC9215246
- DOI: 10.1128/jvi.00508-22
DLK-Dependent Biphasic Reactivation of Herpes Simplex Virus Latency Established in the Absence of Antivirals
Abstract
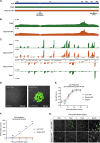
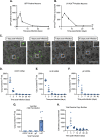
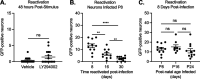
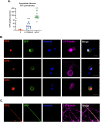
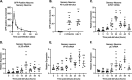
Understanding the molecular mechanisms of herpes simplex virus 1 (HSV-1) latent infection and reactivation in neurons requires the use of in vitro model systems. Establishing a quiescent infection in cultured neurons is problematic, as any infectious virus released can superinfect the cultures. Previous studies have used the viral DNA replication inhibitor acyclovir to prevent superinfection and promote latency establishment. Data from these previous models have shown that reactivation is biphasic, with an initial phase I expression of all classes of lytic genes, which occurs independently of histone demethylase activity and viral DNA replication but is dependent on the cell stress protein DLK. Here, we describe a new model system using HSV-1 Stayput-GFP, a reporter virus that is defective for cell-to-cell spread and establishes latent infections without the need for acyclovir. The establishment of a latent state requires a longer time frame than previous models using DNA replication inhibitors. This results in a decreased ability of the virus to reactivate using established inducers, and as such, a combination of reactivation triggers is required. Using this system, we demonstrate that biphasic reactivation occurs even when latency is established in the absence of acyclovir. Importantly, phase I lytic gene expression still occurs in a histone demethylase and viral DNA replication-independent manner and requires DLK activity. These data demonstrate that the two waves of viral gene expression following HSV-1 reactivation are independent of secondary infection and not unique to systems that require acyclovir to promote latency establishment. IMPORTANCE Herpes simplex virus-1 (HSV-1) enters a latent infection in neurons and periodically reactivates. Reactivation manifests as a variety of clinical symptoms. Studying latency and reactivation in vitro is invaluable, allowing the molecular mechanisms behind both processes to be targeted by therapeutics that reduce the clinical consequences. Here, we describe a novel in vitro model system using a cell-to-cell spread-defective HSV-1, known as Stayput-GFP, which allows for the study of latency and reactivation at the single neuron level. We anticipate this new model system will be an incredibly valuable tool for studying the establishment and reactivation of HSV-1 latent infection in vitro. Using this model, we find that initial reactivation events are dependent on cellular stress kinase DLK but independent of histone demethylase activity and viral DNA replication. Our data therefore further validate the essential role of DLK in mediating a wave of lytic gene expression unique to reactivation.
Keywords: dual leucine zipper kinase; herpes simplex virus; human herpesviruses; in vitro model systems; latent infection; reactivation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




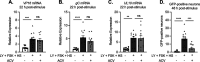
Similar articles
-
Ex Vivo Herpes Simplex Virus Reactivation Involves a Dual Leucine Zipper Kinase-Dependent Wave of Lytic Gene Expression That Is Independent of Histone Demethylase Activity and Viral Genome Synthesis.J Virol. 2022 Jun 22;96(12):e0047522. doi: 10.1128/jvi.00475-22. Epub 2022 May 23. J Virol. 2022. PMID: 35604215 Free PMC article.
-
An Immortalized Human Dorsal Root Ganglion Cell Line Provides a Novel Context To Study Herpes Simplex Virus 1 Latency and Reactivation.J Virol. 2017 May 26;91(12):e00080-17. doi: 10.1128/JVI.00080-17. Print 2017 Jun 15. J Virol. 2017. PMID: 28404842 Free PMC article.
-
Neuronal hyperexcitability is a DLK-dependent trigger of herpes simplex virus reactivation that can be induced by IL-1.Elife. 2020 Dec 22;9:e58037. doi: 10.7554/eLife.58037. Elife. 2020. PMID: 33350386 Free PMC article.
-
[Mechanisms of herpes simplex virus latency and reactivation].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019 May 25;48(1):89-101. doi: 10.3785/j.issn.1008-9292.2019.02.14. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2019. PMID: 31102363 Free PMC article. Review. Chinese.
-
Restarting Lytic Gene Transcription at the Onset of Herpes Simplex Virus Reactivation.J Virol. 2017 Jan 3;91(2):e01419-16. doi: 10.1128/JVI.01419-16. Print 2017 Jan 15. J Virol. 2017. PMID: 27807236 Free PMC article. Review.
Cited by
-
Regulation of the Activity of the Dual Leucine Zipper Kinase by Distinct Mechanisms.Cells. 2024 Feb 11;13(4):333. doi: 10.3390/cells13040333. Cells. 2024. PMID: 38391946 Free PMC article. Review.
-
Impact of Cultured Neuron Models on α-Herpesvirus Latency Research.Viruses. 2022 Jun 2;14(6):1209. doi: 10.3390/v14061209. Viruses. 2022. PMID: 35746680 Free PMC article. Review.
-
A dual fluorescent herpes simplex virus type 1 recombinant reveals divergent outcomes of neuronal infection.J Virol. 2024 May 14;98(5):e0003224. doi: 10.1128/jvi.00032-24. Epub 2024 Apr 23. J Virol. 2024. PMID: 38651900 Free PMC article.
-
Ex Vivo Herpes Simplex Virus Reactivation Involves a Dual Leucine Zipper Kinase-Dependent Wave of Lytic Gene Expression That Is Independent of Histone Demethylase Activity and Viral Genome Synthesis.J Virol. 2022 Jun 22;96(12):e0047522. doi: 10.1128/jvi.00475-22. Epub 2022 May 23. J Virol. 2022. PMID: 35604215 Free PMC article.
-
Lytic promoter activity during herpes simplex virus latency is dependent on genome location.J Virol. 2024 Nov 19;98(11):e0125824. doi: 10.1128/jvi.01258-24. Epub 2024 Oct 21. J Virol. 2024. PMID: 39431845 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

