Type 2 diabetes mellitus accelerates brain aging and cognitive decline: Complementary findings from UK Biobank and meta-analyses
- PMID: 35608247
- PMCID: PMC9132576
- DOI: 10.7554/eLife.73138
Type 2 diabetes mellitus accelerates brain aging and cognitive decline: Complementary findings from UK Biobank and meta-analyses
Abstract
Background: Type 2 diabetes mellitus (T2DM) is known to be associated with neurobiological and cognitive deficits; however, their extent, overlap with aging effects, and the effectiveness of existing treatments in the context of the brain are currently unknown.
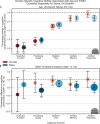
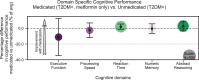
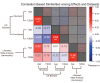
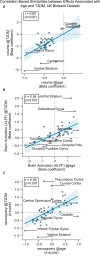
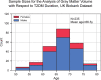
Methods: We characterized neurocognitive effects independently associated with T2DM and age in a large cohort of human subjects from the UK Biobank with cross-sectional neuroimaging and cognitive data. We then proceeded to evaluate the extent of overlap between the effects related to T2DM and age by applying correlation measures to the separately characterized neurocognitive changes. Our findings were complemented by meta-analyses of published reports with cognitive or neuroimaging measures for T2DM and healthy controls (HCs). We also evaluated in a cohort of T2DM-diagnosed individuals using UK Biobank how disease chronicity and metformin treatment interact with the identified neurocognitive effects.
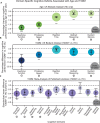
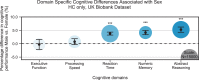
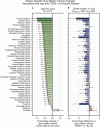
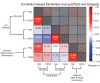
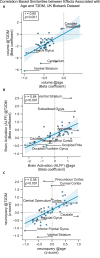
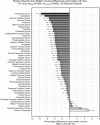
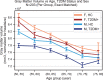
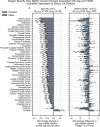
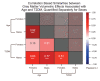
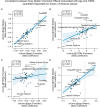
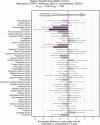
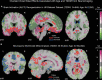
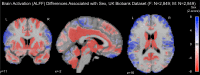
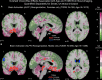
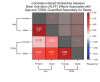
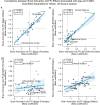
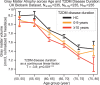
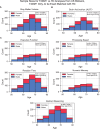
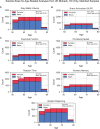
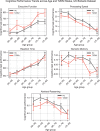
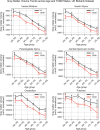
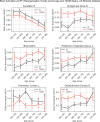
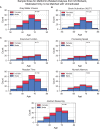
Results: The UK Biobank dataset included cognitive and neuroimaging data (N = 20,314), including 1012 T2DM and 19,302 HCs, aged between 50 and 80 years. Duration of T2DM ranged from 0 to 31 years (mean 8.5 ± 6.1 years); 498 were treated with metformin alone, while 352 were unmedicated. Our meta-analysis evaluated 34 cognitive studies (N = 22,231) and 60 neuroimaging studies: 30 of T2DM (N = 866) and 30 of aging (N = 1088). Compared to age, sex, education, and hypertension-matched HC, T2DM was associated with marked cognitive deficits, particularly in executive functioning and processing speed. Likewise, we found that the diagnosis of T2DM was significantly associated with gray matter atrophy, primarily within the ventral striatum, cerebellum, and putamen, with reorganization of brain activity (decreased in the caudate and premotor cortex and increased in the subgenual area, orbitofrontal cortex, brainstem, and posterior cingulate cortex). The structural and functional changes associated with T2DM show marked overlap with the effects correlating with age but appear earlier, with disease duration linked to more severe neurodegeneration. Metformin treatment status was not associated with improved neurocognitive outcomes.
Conclusions: The neurocognitive impact of T2DM suggests marked acceleration of normal brain aging. T2DM gray matter atrophy occurred approximately 26% ± 14% faster than seen with normal aging; disease duration was associated with increased neurodegeneration. Mechanistically, our results suggest a neurometabolic component to brain aging. Clinically, neuroimaging-based biomarkers may provide a valuable adjunctive measure of T2DM progression and treatment efficacy based on neurological effects.
Funding: The research described in this article was funded by the W. M. Keck Foundation (to LRMP), the White House Brain Research Through Advancing Innovative Technologies (BRAIN) Initiative (NSFNCS-FR 1926781 to LRMP), and the Baszucki Brain Research Fund (to LRMP). None of the funding sources played any role in the design of the experiments, data collection, analysis, interpretation of the results, the decision to publish, or any aspect relevant to the study. DJW reports serving on data monitoring committees for Novo Nordisk. None of the authors received funding or in-kind support from pharmaceutical and/or other companies to write this article.
Keywords: MRI; aging; brain; diabetes; epidemiology; functional MRI; global health; human; medicine; neuroimaging.
© 2022, Antal et al.
Conflict of interest statement
BA, LM, SS, AL, LM No competing interests declared, DW is part of a Novo Nordisk data monitoring committee service for semaglutide in SOUL and FLOW trials. The author has no other competing interests to declare, BD received royalties from Cambridge University Press and Oxford University Press, and consulting fees from Acadia, Alector, Arkuda, Biogen, Denali, Lilly, Merck, Novartis, Takeda, Wave LifeSciences (unrelated to the present work). Also participates in a Lilly Data Safety Monitoring Board (unrelated to the present work) and participates in leadership roles in Alzheimer's Association and Association for Frontotemporal Degeneration. The author has no other competing interests to declare, ER received honoraria from Harvard Catalyst. The author has no other competing interests to declare
Figures
Similar articles
-
Multimodality neuroimaging brain-age in UK biobank: relationship to biomedical, lifestyle, and cognitive factors.Neurobiol Aging. 2020 Aug;92:34-42. doi: 10.1016/j.neurobiolaging.2020.03.014. Epub 2020 Apr 8. Neurobiol Aging. 2020. PMID: 32380363 Free PMC article.
-
Association between Type 2 Diabetes Mellitus and Brain Atrophy: A Meta-Analysis.Diabetes Metab J. 2022 Sep;46(5):781-802. doi: 10.4093/dmj.2021.0189. Epub 2022 Mar 8. Diabetes Metab J. 2022. PMID: 35255549 Free PMC article.
-
Abnormal changes of brain function and structure in patients with T2DM-related cognitive impairment: a neuroimaging meta-analysis and an independent validation.Nutr Diabetes. 2024 Nov 11;14(1):91. doi: 10.1038/s41387-024-00348-5. Nutr Diabetes. 2024. PMID: 39528442 Free PMC article. Review.
-
Repaglinide : a pharmacoeconomic review of its use in type 2 diabetes mellitus.Pharmacoeconomics. 2004;22(6):389-411. doi: 10.2165/00019053-200422060-00005. Pharmacoeconomics. 2004. PMID: 15099124 Review.
-
[A review on the application of UK Biobank in neuroimaging].Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021 Jun 25;38(3):594-601. doi: 10.7507/1001-5515.202012059. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2021. PMID: 34180206 Free PMC article. Review. Chinese.
Cited by
-
A systematic review of the impact of type 2 diabetes on brain cortical thickness.Front Dement. 2024 Jun 13;3:1418037. doi: 10.3389/frdem.2024.1418037. eCollection 2024. Front Dement. 2024. PMID: 39081608 Free PMC article.
-
Diet and lifestyle impact the development and progression of Alzheimer's dementia.Front Nutr. 2023 Jun 29;10:1213223. doi: 10.3389/fnut.2023.1213223. eCollection 2023. Front Nutr. 2023. PMID: 37457976 Free PMC article. Review.
-
Associations of plasma proteomics and age-related outcomes with brain age in a diverse cohort.Geroscience. 2024 Aug;46(4):3861-3873. doi: 10.1007/s11357-024-01112-4. Epub 2024 Mar 4. Geroscience. 2024. PMID: 38438772 Free PMC article.
-
Systemic inflammation attenuates the repair of damaged brains through reduced phagocytic activity of monocytes infiltrating the brain.Mol Brain. 2024 Jul 29;17(1):47. doi: 10.1186/s13041-024-01116-3. Mol Brain. 2024. PMID: 39075534 Free PMC article.
-
[Cognition and depression in older people with diabetes].Nervenarzt. 2024 Jan;95(1):46-52. doi: 10.1007/s00115-023-01599-w. Epub 2024 Jan 8. Nervenarzt. 2024. PMID: 38189938 Review. German.
References
-
- Alfaro-Almagro F, Jenkinson M, Bangerter NK, Andersson JLR, Griffanti L, Douaud G, Sotiropoulos SN, Jbabdi S, Hernandez-Fernandez M, Vallee E, Vidaurre D, Webster M, McCarthy P, Rorden C, Daducci A, Alexander DC, Zhang H, Dragonu I, Matthews PM, Miller KL, Smith SM. Image processing and Quality Control for the first 10,000 brain imaging datasets from UK Biobank. NeuroImage. 2018;166:400–424. doi: 10.1016/j.neuroimage.2017.10.034. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

