Lymphocytes in tumor-draining lymph nodes co-cultured with autologous tumor cells for adoptive cell therapy
- PMID: 35606862
- PMCID: PMC9125345
- DOI: 10.1186/s12967-022-03444-1
Lymphocytes in tumor-draining lymph nodes co-cultured with autologous tumor cells for adoptive cell therapy
Abstract
Background: Tumor-draining lymph nodes (TDLNs) are primary sites, where anti-tumor lymphocytes are primed to tumor-specific antigens and play pivotal roles in immune responses against tumors. Although adoptive cell therapy (ACT) using lymphocytes isolated from TDLNs were reported, characterization of immune activity of lymphocytes in TDLNs to tumor cells was not comprehensively performed. Here, we demonstrate TDLNs to have very high potential as cell sources for immunotherapy.
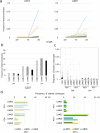
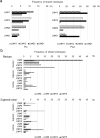
Methods: Lymphocytes from TDLNs resected during surgical operation were cultured with autologous-tumor cells for 2 weeks and evaluated tumor-reactivity by IFNγ ELISPOT assay. We investigated the commonality of T cell receptor (TCR) clonotypes expanded by the co-culture with tumor cells with those of tumor infiltrating lymphocytes (TILs).
Results: We found that that TCR clonotypes of PD-1-expressing CD8+ T cells in lymph nodes commonly shared with those of TILs in primary tumors and lymphocytes having tumor-reactivity and TCR clonotypes shared with TILs could be induced from non-metastatic lymph nodes when they were co-cultured with autologous tumor cells.
Conclusion: Our results imply that tumor-reactive effector T cells were present even in pathologically non-metastatic lymph nodes and could be expanded in vitro in the presence of autologous tumor cells and possibly be applied for ACT.
Keywords: Adoptive T cell therapy; Colorectal cancer; Tumor-draining lymph nodes; Tumor-infiltrating lymphocytes.
© 2022. The Author(s).
Conflict of interest statement
Kazuma Kiyotani reported advisory roles to Cancer Precision Medicine Inc, Japan. Yusuke Nakamura is a stockholder and an advisor of OncoTherapy Science, Inc, Japan.
Figures

Similar articles
-
CD4+ and CD8+ T cells in sentinel nodes exhibit distinct pattern of PD-1, CD69, and HLA-DR expression compared to tumor tissue in oral squamous cell carcinoma.Cancer Sci. 2021 Mar;112(3):1048-1059. doi: 10.1111/cas.14816. Epub 2021 Feb 15. Cancer Sci. 2021. PMID: 33462898 Free PMC article.
-
TCR sequencing analysis of cancer tissues and tumor draining lymph nodes in colorectal cancer patients.Oncoimmunology. 2019 Mar 22;8(6):e1588085. doi: 10.1080/2162402X.2019.1588085. eCollection 2019. Oncoimmunology. 2019. PMID: 31069156 Free PMC article.
-
Neoantigen-specific stem cell memory-like CD4+ T cells mediate CD8+ T cell-dependent immunotherapy of MHC class II-negative solid tumors.Nat Immunol. 2023 Aug;24(8):1345-1357. doi: 10.1038/s41590-023-01543-9. Epub 2023 Jul 3. Nat Immunol. 2023. PMID: 37400675 Free PMC article.
-
Adoptive CD8+ T cell therapy against cancer:Challenges and opportunities.Cancer Lett. 2019 Oct 10;462:23-32. doi: 10.1016/j.canlet.2019.07.017. Epub 2019 Jul 26. Cancer Lett. 2019. PMID: 31356845 Review.
-
Activation of T lymphocytes for the adoptive immunotherapy of cancer.Ann Surg Oncol. 1994 Jul;1(4):296-306. doi: 10.1007/BF02303568. Ann Surg Oncol. 1994. PMID: 7850528 Review.
Cited by
-
Harnessing novel strategies and cell types to overcome immune tolerance during adoptive cell therapy in cancer.J Immunother Cancer. 2023 Apr;11(4):e006434. doi: 10.1136/jitc-2022-006434. J Immunother Cancer. 2023. PMID: 37100458 Free PMC article. Review.
-
Characterization of double-negative T cells in colorectal cancers and their corresponding lymph nodes.Oncoimmunology. 2024 Jul 4;13(1):2373530. doi: 10.1080/2162402X.2024.2373530. eCollection 2024. Oncoimmunology. 2024. PMID: 38979545 Free PMC article.
-
Tumor-draining lymph nodes: opportunities, challenges, and future directions in colorectal cancer immunotherapy.J Immunother Cancer. 2024 Jan 19;12(1):e008026. doi: 10.1136/jitc-2023-008026. J Immunother Cancer. 2024. PMID: 38242718 Free PMC article. Review.
-
IL-7R licenses a population of epigenetically poised memory CD8+ T cells with superior antitumor efficacy that are critical for melanoma memory.Proc Natl Acad Sci U S A. 2023 Jul 25;120(30):e2304319120. doi: 10.1073/pnas.2304319120. Epub 2023 Jul 17. Proc Natl Acad Sci U S A. 2023. PMID: 37459511 Free PMC article.
-
Prognostic effect of TCF1+ CD8+ T cell and TOX+ CD8+ T cell infiltration in lung adenocarcinoma.Cancer Sci. 2024 Jul;115(7):2184-2195. doi: 10.1111/cas.16177. Epub 2024 Apr 9. Cancer Sci. 2024. PMID: 38590234 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

